��Ŀ����
����Ŀ��Ϊ���������Ⱦ������Ҫ��ǿ�Թ�ҵ����������β����������������ѧ֪ʶ�ش��������⣺
��1����ʯȼ�ϰ���ú��ʯ�ͺ�___��
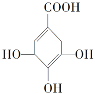
��2��������ָpH___(���������������)5.6�Ľ�ˮ��ú��ȼ���ǵ��������γɵ���Ҫԭ��������ˮ��pHԼΪ5.6��ԭ����___(�û�ѧ����ʽ��ʾ)��
��3��������β���ŷſڼ�װ����Ч�������������ڲ������������ʵ�����£��ɽ�β���е�һ����̼��һ������ת��Ϊ�������ѭ����������������壬�÷�Ӧ�Ļ�ѧ����ʽΪ___��
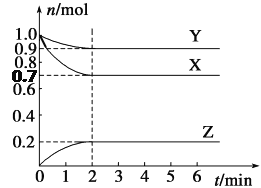
��4��������Դ����δ�ռ�ʱ����ͼ��ʾΪһ�֡����ܼ��š�����Ч��ʩ�����¶��Ҵ�������ȼ�ϵ������������____(����ĸ����ͬ)��

A.ԭ����Դ�ḻ B.�ǿ�������Դ C.ȼ����ȫû����Ⱦ
��5��Cu��ϡHNO3��Ӧ�����ӷ���ʽΪ��___��
��6����ҵ�Ͻ�����ͨ��ʯ������ȡƯ�ۣ�Ư�۵���Ч�ɷ���___��
��7��������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�NO2��NO��2NaOH=2NaNO2��H2O��2NO2��2NaOH=NaNO2��NaNO3��H2O������VLijNaOH��Һ����ȫ����nmolNO2��mmolNO��ɵĴ�����Ⱦ�
�������ռ���Һ�����ʵ���Ũ������Ϊ____mol��L-1��
����������Һ��c(NO3-)��c(NO2-)=1��9����ԭ���������NO2��NO�����ʵ���֮��n��m=____��
���𰸡���Ȼ�� �� CO2+H2O![]() H2CO3 2NO+2CO
H2CO3 2NO+2CO N2+2CO2 C 3Cu+8H++2NO3-=3Cu2++2NO��+4H2O Ca(ClO)2
N2+2CO2 C 3Cu+8H++2NO3-=3Cu2++2NO��+4H2O Ca(ClO)2 ![]() 3��2
3��2
��������
(1)ú��ʯ�ͺ���Ȼ����ĿǰӦ�����Ļ�ʯȼ�ϣ��ʴ�Ϊ����Ȼ����
(2)������ָpHС��5.6����ˮ����������ˮ���ܽ��˿����еĶ�����̼�����������ԣ�ԭ���Ƕ�����̼��ˮ��Ӧ������̼�ᣬ��ӦΪCO2+H2O![]() H2CO3��̼�������ԣ��ʴ�Ϊ������CO2+H2O
H2CO3��̼�������ԣ��ʴ�Ϊ������CO2+H2O![]() H2CO3��
H2CO3��
(3)β���е�һ����̼��һ������ת��Ϊ�������ѭ����������������壬��֪��������Ϊ������̼�͵������ɴ˿ɵ÷�Ӧ����ʽΪ2NO+2CO N2+2CO2���ʴ�Ϊ��2NO+2CO
N2+2CO2���ʴ�Ϊ��2NO+2CO N2+2CO2��
N2+2CO2��
(4)A.��ҵ�Ҵ����Ե��ۻ�����ά��ˮ���õ������ǣ������Ƿ��͵õ��Ҵ��������ۺ���ά�ؿ��Դ�ֲ���л�ã���Դ�ḻ����A��ȷ��
B.��A������֪�Ҵ�����������ֲ�ֲ���ǿ������ģ�����Ҵ��ǿ�������Դ��B��ȷ��
C.�Ҵ�ȼ��ʱ����������̼��ͬʱҲ�ɻ���ڲ���ȫȼ�ղ���һ����̼��������̼�Ĵ����ŷŻᵼ������ЧӦ��һ����̼�ж�������Ҵ�ȼ��Ҳ��Ի�������Ⱦ����C����
��ѡC��
(5)ͭ��ϡ���ᷴӦ��������ͭ�����ᱻ��ԭ��һ���������壬��Ӧ�����ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
(6)����ͨ��ʯ���鷴Ӧ�����Ȼ��ƺʹ�����ƣ�����ΪƯ�۵���Ҫ�ɷ֣����д�����ƾ���ǿ��������Ư�۵���Ч�ɷ֣��ʴ�Ϊ��Ca(ClO)2��
(7)��NO2��NO��2NaOH=2NaNO2��H2O��2NO2��2NaOH=NaNO2��NaNO3��H2O���ɷ�Ӧ��֪����Ӧ��������������������ʵ���֮�Ⱦ�Ϊ1:1,�����ȫ����nmolNO2��mmolNOʱ�����������Ƶ����ʵ���������������ʵ�������n��NaOH��=n��NO2��+n��NO��=��m+n��mol�������ռ���Һ�����ʵ���Ũ��=![]() ,�ʴ�Ϊ��
,�ʴ�Ϊ��![]() ��
��
����������Һ��c(NO3-)��c(NO2-)=1��9������������ʵ���Ϊ1mol��������������ʵ���Ϊ9mol���ɷ�Ӧ2NO2��2NaOH=NaNO2��NaNO3��H2O��֪���÷�Ӧ�������������Ƶ��������Ƶ����ʵ�������ҲΪ1mol���÷�Ӧ�����ĵĶ�������Ϊ2mol����NO2��NO��2NaOH=2NaNO2��H2O��������������Ϊ9mol-1mol=8mol���÷�Ӧ�����ĵĶ���������һ��������Ϊ4mol���ɴ˿ɵö�����������Ϊ2mol+4mol=6mol��һ������Ϊ4mol��ԭ���������NO2��NO�����ʵ���֮��3:2���ʴ�Ϊ��3:2��

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�