��Ŀ����
(16��)ij�о�С�����ô�ʳ��(��Ca2+��Mg2+��SO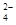 ��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�

�Իش��������⣺
��1����ҵ��һ�������ù�����̿�ڸ����»�ԭʯӢɰ����ȡ�ֹ裬д���ù��̵Ļ�ѧ����ʽ��_______________________________________________________________________��
��2�����ƴ���ˮ�����Լ�Ϊ��BaC12����Na2CO3����HC1����NaOH����μӵ��Ⱥ�˳�������е�________(�����и�������)��
a���٢ڢܢ� b���ܢڢ٢� c���ܢ٢ۢ� d���ڢܢ٢�
��֪��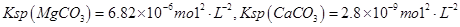 ������ô���ˮ��
������ô���ˮ��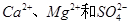 ��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��3����֪SiCl4�ķе���57.6�棬CC14�ķе���76.8�档�ڷ�Ӧ��I�еõ���SiCl4��Ʒ�к���CCl4�����еõ�����SiCl4�ɲ��õķ��������и����е�________(�����)��
a������ b������ c����Һ d������
��Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
��4����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ʾ��ͼ������������������������_____����ƷA�Ļ�ѧʽΪ____________��

��������Ĥ���۵�ⱥ��ʳ��ˮ����ȡ�������ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ__ ___��
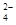 ��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
�Իش��������⣺
��1����ҵ��һ�������ù�����̿�ڸ����»�ԭʯӢɰ����ȡ�ֹ裬д���ù��̵Ļ�ѧ����ʽ��_______________________________________________________________________��
��2�����ƴ���ˮ�����Լ�Ϊ��BaC12����Na2CO3����HC1����NaOH����μӵ��Ⱥ�˳�������е�________(�����и�������)��
a���٢ڢܢ� b���ܢڢ٢� c���ܢ٢ۢ� d���ڢܢ٢�
��֪��
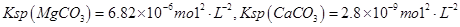 ������ô���ˮ��
������ô���ˮ��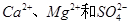 ��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________����3����֪SiCl4�ķе���57.6�棬CC14�ķе���76.8�档�ڷ�Ӧ��I�еõ���SiCl4��Ʒ�к���CCl4�����еõ�����SiCl4�ɲ��õķ��������и����е�________(�����)��
a������ b������ c����Һ d������
��Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
��4����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ʾ��ͼ������������������������_____����ƷA�Ļ�ѧʽΪ____________��

��������Ĥ���۵�ⱥ��ʳ��ˮ����ȡ�������ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ__ ___��
��16�֣�
��1��SiO2+2C Si+2CO����2�֣�
Si+2CO����2�֣�
��2��a��2�֣���̼��ƣ���CaCO3����2�֣�
��3��a��2�֣���SiCl4+2H2 Si+4HCl��2�֣�
Si+4HCl��2�֣�
��4����������H2����2�֣���NaOH��2�֣���NaCl+H2O NaClO+H2����2�֣�
NaClO+H2����2�֣�
��1��SiO2+2C
 Si+2CO����2�֣�
Si+2CO����2�֣���2��a��2�֣���̼��ƣ���CaCO3����2�֣�
��3��a��2�֣���SiCl4+2H2
 Si+4HCl��2�֣�
Si+4HCl��2�֣���4����������H2����2�֣���NaOH��2�֣���NaCl+H2O
 NaClO+H2����2�֣�
NaClO+H2����2�֣������������1��C�ڸ��������»�ԭSiO2������Si��CO����ѧ����ʽΪ��SiO2+2C
 Si+2CO��
Si+2CO����2��Na2CO3��Һ������Ϊ��ȥCa2+����ȥ������BaCl2��Һ������Na2CO3��˳����BaCl2�ĺ��棬HCl�������dz�ȥ������Na2CO3��NaOH��������������a����ȷ����ΪKsp(MgCO3) < Ksp(CaCO3)��CaCO3�����ܣ��������������ij�����CaCO3��
��3��SiCl4��CCl4�ڳ�����ΪҺ�壬��ܽ⣬���е㲻ͬ������������ķ����õ�������SiCl4����Ӧ��II��H2��ԭSiCl4����ѧ����ʽΪ��SiCl4+2H2
 Si+4HCl��
Si+4HCl����4�����ݷŵ�˳��������H2O�������H+�ŵ磬���Ե���������������������������ˮ�������H+�ŵ磬�ٽ�H2O�ĵ���ƽ�������ƶ���OH?Ũ���������Բ�ƷAΪNaOH����Ĥ���۵�ⱥ��ʳ��ˮ����ȡ�������ƣ���������Cl2��NaOH��Ӧ���ɴ������ƣ����Ի�ѧ����ʽΪ��NaCl+H2O
 NaClO+H2��
NaClO+H2��
��ϰ��ϵ�д�
�����Ŀ