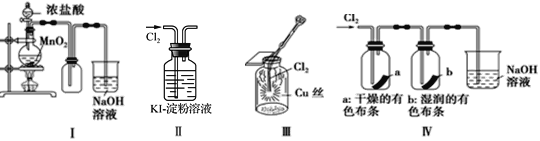
��Ŀ����
����Ŀ����ˮ�Ǿ����Դ���⣬��ˮ���Ȼ���ĺ����൱�ߣ���Ҫ���Ȼ��ƣ�������Ȼ�þ���Ȼ��ƺ��Ȼ��صȡ��Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
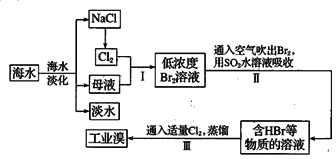
(1)��ԭ�ӵĽṹʾ��ͼΪ____________________��
(2)��NaClΪԭ�����������Ļ�ѧ����ʽΪ___________________________________��
(3)����������ȿ����Ϳ���Br2��������ԭ����_____________________________��
(4)���������SO2ˮ��Һ����Br2�����Ƶ�HBr�����ᣬ��д���÷�Ӧ�Ļ�ѧ����ʽ_________________________________________________��
(5)�������������ȡ�壬����������������__________�ԡ�������������������ԭ������
���𰸡�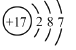 2NaCl+2H2O
2NaCl+2H2O![]() 2NaOH+H2��+Cl2�� Br2�ӷ� Br2+SO2+2H2O=2HBr+H2SO4 ����
2NaOH+H2��+Cl2�� Br2�ӷ� Br2+SO2+2H2O=2HBr+H2SO4 ����
��������
���ȡ���ȵ����ʣ�������ˮ��Դ�ۺ����õ�ԭ����
(1)����17��Ԫ�أ��ɺ�������Ų����ɣ���д����ԭ�ӽṹʾ��ͼ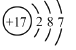 ��
��
(2)��NaClΪԭ������������ͨ���õ�ⱥ��ʳ��ˮ������ѧ����ʽΪ2NaCl+2H2O![]() 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����
(3)Br2�ӷ����ȿ���ͨ�뺬Br2�ĺ�ˮ�пɽ��䴵����
(4)��SO2ˮ��Һ����Br2����HBr�����ᣬ��д����Ӧ�������Ļ�ѧʽ����ƽ���÷�Ӧ�Ļ�ѧ����ʽBr2+SO2+2H2O=2HBr+H2SO4��
(5)�������������ȡ�壬������ӦCl2+2HBr��Br2+2HCl�����������������÷�Ӧ�����������������ԡ�

����Ŀ��̼�������仯�����ڹ�ũҵ����������������Ҫ���á���ش��������⣺
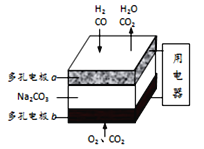
��1������β���еĴ���NO�ķ���Ҳ����H2��NO��ԭΪN2��
��֪��
H2��ԭNO���ɵ�����ˮ�������Ȼ�ѧ������_______________________________��
��2����¯���������ĸ�¯���к���CO��H2��CO2�����壬����CO��H2�ڴ��������ºϳɼ״����Ǽ�����Ⱦ����Լ��Դ��һ���¾ٴ룬��Ӧԭ��ΪCO(g)+2H2(g)![]() CH3OH(g) ��H���������ͬ�����������ܱ������зֱ����1molCO��2mol H2�����ƽ��������CH3OH ����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��
CH3OH(g) ��H���������ͬ�����������ܱ������зֱ����1molCO��2mol H2�����ƽ��������CH3OH ����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��

�������ϳɼ״��ķ�Ӧ��__________________������ȡ����ȡ�����Ӧ��ͼ���е�ѹǿp1��p2�Ĵ�С��ϵ��_______________���жϵ�������________________________��
�ڴ���ͼA��B��C������ѡ���±���������Ӧ���ĵ㣨�á�A������B����C����д����
��Ӧ����V | ƽ�ⳣ��K | ƽ��ת����a |
____________ | _____________ | _____________ |
����300��ʱ����C��ƽ����ϵ���ٳ���0.25molCO��0.5molH2��0.25molCH3OH����ƽ��_________���������Ӧ���������淴Ӧ���������ƶ���
��3�����������COƽ��ת���ʵĴ�ʩ��_________________��
A.ʹ�ô��� B.Ͷ�ϱȲ��䣬����CO��Ũ��
C.���ͷ�Ӧ�¶� D.ͨ��He����ʹ��ϵ��ѹǿ����
��4��һ���¶��£�CO��ת��������ʼͶ�ϱ�![]() �ı仯��ϵ��ͼ��ʾ�����D��������ת����Ϊ40%����x=__________________��
�ı仯��ϵ��ͼ��ʾ�����D��������ת����Ϊ40%����x=__________________��