��Ŀ����
����Ŀ������̼������ʱ�У�Ҳ�ǻ���Ҫ������̼���ڹ�ҵ�����������ش������ԭ���ϣ����ŷŻ�����ŷ�������������ͬ����������������ȶ��Ǻܺõ�����̼��������ʽ�������Ǽ����������÷���������(Һ)����������������淋Ĺ��գ�
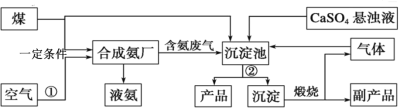
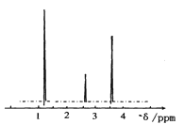
��ش��������⣺����ʾ��1��ú��һ����������ȡ��ҵú������Ҫ��H2��CO2�����壻2��������ͨ��������Һ������������O2���N2��
��1�����ղ�����Ϊ��_____��
��2��ʵ�����ü��ȹ�������ķ����Ʊ������Ļ�ѧ����ʽΪ��_____��
��3������Ʒ�Ļ�ѧʽΪ_____�����������������п���ѭ��ʹ�õ�������_____��
��4����ʵ�����м��鰱���ķ�����_____��
��5��д����������Ʒ���Ļ�ѧ����ʽ��_____��
���𰸡����� Ca(OH)2��2NH4Cl![]() 2NH3����CaCl2��2H2O CaO CO2 ��ʪ���ɫʯ����ֽ�����Թܣ���ֽ������֤���а��� CaSO4��CO2��2NH3��H2O=(NH4)2SO4��CaCO3
2NH3����CaCl2��2H2O CaO CO2 ��ʪ���ɫʯ����ֽ�����Թܣ���ֽ������֤���а��� CaSO4��CO2��2NH3��H2O=(NH4)2SO4��CaCO3
��������
�����̿�֪��ú���еĶ�����̼��ϳɰ��ķ���һ��ͨ���������Һ�У����������������Һ��̼��Ƴ��������˺�̼��Ƹ������տɵõ�����ƷCaO�Ͷ�����̼���壬������̼����ѭ�����á�
��1�����ղ�����Ϊ����̼��Ƴ������������Һ�IJ���������Ϊ���ˡ�
��2��ʵ�����ü����Ȼ�狀��������ƵĹ�������ķ����Ʊ���������ѧ����ʽΪCa(OH)2��2NH4Cl![]() 2NH3����CaCl2��2H2O��
2NH3����CaCl2��2H2O��
��3������̼��Ƶõ��Ĺ���Ϊ����Ʒ����ͼ����Ϣ��֪���仯ѧʽΪCaO������̼��Ƶõ���������CO2�����ڣ��ڳ���������CO2���뷴Ӧ���ʸ��������������п���ѭ��ʹ�õ�������CO2��
��4��������ˮ��Һ�Լ��� ����ʵ�����м��鰱���ķ����ǣ���ʪ���ɫʯ����ֽ�����Թܣ���ֽ������֤���а�����
��5��������̼��ϳɰ��ķ���һ��ͨ���������Һ�У����������������Һ��̼��Ƴ����� ����Ʒ��ָ��������泥��÷�Ӧ�Ļ�ѧ����ʽΪCaSO4��CO2��2NH3��H2O=(NH4)2SO4��CaCO3��

����Ŀ��ij̽����ѧϰС������H2C2O4��Һ������KMnO4��Һ֮��ķ�Ӧ��̽����������ı�Ի�ѧ��Ӧ���ʵ�Ӱ�졣ʵ�������ʾ�������й�˵������ȷ���ǣ� ��
ʵ�� ��� | ʵ���¶�/K | ����KMnO4��Һ | H2C2O4��Һ | H2O | ��Һ������ɫʱ����ʱ��/s | ||
V/mL | c/mol��L-1 | V/mL | c/mol��L-1 | V/mL | |||
A | 293 | 2 | 0.02 | 5 | 0.1 | 3 | t1 |
B | T1 | 2 | 0.02 | 3 | 0.1 | V1 | 8 |
C | 313 | 2 | 0.02 | V2 | 0.1 | 5 | t1 |
A.����KMnO4��Һʱ����ϡ�����ữ
B.��t1<8�����ͨ�����Ʊ�������ʵ��A��B̽��H2C2O4Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�첢�ó����ۣ�����Ӧ��Ũ�ȣ���ѧ��Ӧ���ʼӿ�
C.��span>T1=293K��V1 =V2=3mL�����ͨ��ʵ��B��C̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ��
D.����ʵ��B�����ݼ��㣬��KMnO4��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ����v��KMnO4��=5��10-4mol��L-1��s-1