��Ŀ����
����Ŀ�������仯������;�dz��㷺���ش��������⣺
(1)��̬Fԭ�ӵļ۲���ӵĹ������ʽΪ____��
(2)[H2F]+[SbF6]-(������)��һ�ֳ�ǿ�ᣬ����[H2F]+�������ӵĿռ乹��Ϊ_____����[H2F]+������ͬ�ռ乹�ͺͼ�����ʽ�ķ��Ӻ������ӷֱ���_____��_____(����һ��)��
(3)NH4F(�����)�����ڲ�����ʴ�̷���������������![]() ������ԭ�ӵ��ӻ�������_____��������д��ڵĻ�ѧ����_____(����ĸ)��
������ԭ�ӵ��ӻ�������_____��������д��ڵĻ�ѧ����_____(����ĸ)��
A.���Ӽ� B.���� C.���� D.���
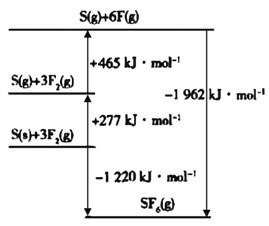
(4)SF6���㷺������ѹ�����豸�ľ�Ե���ʡ�SF6��һ�ֹ��ۻ������ͨ��������Born��Haberѭ��������������ͼ������ؼ��ܡ���F��F���ļ���Ϊ____kJ��mol-1��S��F���ļ���Ϊ____kJ��mol-1��
���𰸡�![]() V�� H2O
V�� H2O ![]() sp3 AB 155 327
sp3 AB 155 327
��������
ͨ���۲���Ӷ������ж�����ԭ�ӵ��ӻ���ʽ�����ӵļ��ι��ͣ���ͼ�ɵã�3F2(g)=6F(g)��H=+465 kJ��mol-1����F-F���ļ���Ϊ465 kJ��mol-1��3=155 kJ��mol-1��ͬ����S-F�ļ��ܡ�
(1)F��ԭ������Ϊ9����������Ų�ʽΪ1s22s22p5���۵���Ϊ2s22p5����˻�̬Fԭ�ӵļ۵����Ų�ͼΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2) [H2F]+����������ԭ��F�ŵ��Ӷ���=![]() ���������Ӷ���Ϊ2���۲���Ӷ���Ϊ4��VSEPRģ��Ϊ�������ͣ��ռ乹��ΪV�ͣ���[H2F]+������ͬ�ռ乹�ͺͼ�����ʽ�ķ��Ӻ������ӷֱ���H2O��
���������Ӷ���Ϊ2���۲���Ӷ���Ϊ4��VSEPRģ��Ϊ�������ͣ��ռ乹��ΪV�ͣ���[H2F]+������ͬ�ռ乹�ͺͼ�����ʽ�ķ��Ӻ������ӷֱ���H2O��![]() ���ʴ�Ϊ��V�ͣ�H2O��
���ʴ�Ϊ��V�ͣ�H2O��![]() ��
��
(3)![]() ��Nԭ���ӻ������Ϊ4+
��Nԭ���ӻ������Ϊ4+![]() ��N��ȡsp3�ӻ����������笠������������֮��������Ӽ���笠������е�ԭ������ԭ���γɵĵ����Ϊ������������˫����û��������ͬʱҲ������������ʴ�ѡAB���ʴ�Ϊ��sp3��AB��
��N��ȡsp3�ӻ����������笠������������֮��������Ӽ���笠������е�ԭ������ԭ���γɵĵ����Ϊ������������˫����û��������ͬʱҲ������������ʴ�ѡAB���ʴ�Ϊ��sp3��AB��
(4)��ͼ�ɵã�3F2(g)=6F(g)��H=+465 kJ��mol-1����F-F���ļ���Ϊ465 kJ��mol-1��3=155 kJ��mol-1��6F(g)+S(g)=SF6(g)����H=-1962 kJ��mol-1����S-F���ļ���Ϊ1962kJ��mol-1��6=327 kJ��mol-1�����ʴ�Ϊ��155��327��