��Ŀ����
�����Ϊl L���ܱ������У�����lmol CO2��3mol H2��һ�������·�����Ӧ��
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) + 49.0 kJ
CH3OH(g)+H2O(g) + 49.0 kJ
���CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
��1���ӷ�Ӧ��ʼ����10 min��������ƽ����Ӧ����v(H2)= �������ʱ���ڣ���Ӧ�ų�������=
��2���ڸ������£���Ӧ��ƽ�ⳣ��K��ֵ = ������2λС�����������ijһʱ�̱����¶Ȳ��䣬ֻ�ı�Ũ�ȣ�ʹc(CO2) =1.00 mol/L��c(H2) = 0.40 mol/L��c(CH3OH) = c(H2O) = 0.80 mol/L����ƽ�� ����д��ţ���
a��������Ӧ�����ƶ� b�����淴Ӧ�����ƶ�
c�����ƶ� d����ȷ��ƽ���ƶ�����
��3�����д�ʩ����ʹn(CH3OH)/n(CO2)������� ����д��ţ���
a�������¶� b������He(g)��ʹ��ϵѹǿ����
c����H2O(g)����ϵ�з��� d���ٳ���l mol CH3OH(g)
��4���ڴ����������£�CO2��H2�ܹ��ϳ��������ʣ���״������࣮�����ѵȣ�����Ӧ��ϵ�л������ʣ���Ӧ���ѽ��У����ܵ�ԭ���� ��
��1��0.225 mol/L?min��2�֣���λ��д��1�֣� 36.75kJ ��1�֣�
��2��5.3��1�֣� b��1�֣�
��3��cd��2�֣�
��4�������ж���1�֣�

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�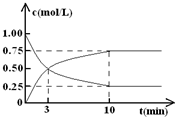 ��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������̼��Դ���о��Ե���Ϊ���ȣ�
��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������̼��Դ���о��Ե���Ϊ���ȣ�
 CH3OH��g��+H2O��g����H=-��n-m��kJ?mol-1
CH3OH��g��+H2O��g����H=-��n-m��kJ?mol-1
 CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011
CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011
 ���¶����ߣ�Kֵ
���¶����ߣ�Kֵ
 CH3OH��g��+H2O��g��
CH3OH��g��+H2O��g�� CH3OH(g)+H2O(g)
��H����49.0 kJ/mol��
CH3OH(g)+H2O(g)
��H����49.0 kJ/mol��
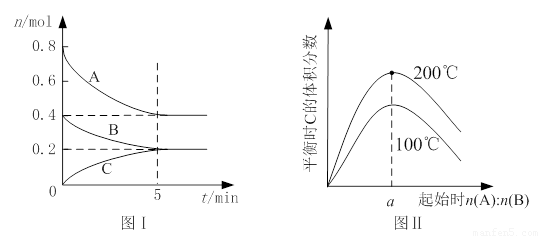
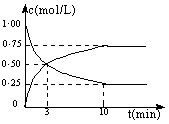
 zC(g)��ͼI��ʾ200��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ���ʾ��ͬ�¶���ƽ��ʱC�������������ʼn(A):n(B)�ı仯��ϵ�������н�����ȷ����
zC(g)��ͼI��ʾ200��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ���ʾ��ͬ�¶���ƽ��ʱC�������������ʼn(A):n(B)�ı仯��ϵ�������н�����ȷ����