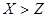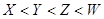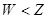��Ŀ����
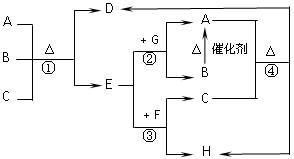
��16�֣�A��F����ѧ��ѧ�г������ʣ�������A��C��E��FΪ���壬B��DΪҺ�壬����B�ķ���Ϊ4ԭ�ӷ��ӣ�D�ڳ����²����лӷ��ԡ�F��Ũ��Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ����Щ����֮��������ͼ��ʾ��ת����ϵ��ͼ�в�������������ȥ��
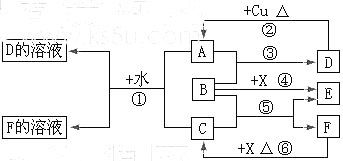
��1��д���������ʵĻ�ѧʽ��A ��F ��
��2��B�ĵ���ʽΪ ������ͼ����Ϣ��B��C��X����������ǿ������˳���� ���û�ѧʽ��ʾ����
��3��д����Ӧ�ٵ����ӷ���ʽ ��
д����Ӧ�����ӷ���ʽ ��
��4���ڷ�Ӧ���У�F���ֵ������� ��������0.75 mol Cʱ���������Ļ�ԭ�������ʵ����� ��

��1��д���������ʵĻ�ѧʽ��A ��F ��
��2��B�ĵ���ʽΪ ������ͼ����Ϣ��B��C��X����������ǿ������˳���� ���û�ѧʽ��ʾ����
��3��д����Ӧ�ٵ����ӷ���ʽ ��
д����Ӧ�����ӷ���ʽ ��
��4���ڷ�Ӧ���У�F���ֵ������� ��������0.75 mol Cʱ���������Ļ�ԭ�������ʵ����� ��
��1�� SO2 HCl (��2��)
��2�� (2��)
(2��)
MnO2��Cl2��H2O2 (2��)
��3����Ӧ�٣�Cl2+SO2+2H2O= 4H++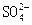 +2Cl�� (2��)
+2Cl�� (2��)
��Ӧ�ޣ� MnO2+4H++2Cl��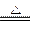 Mn2++Cl2��+2H2O (2��)
Mn2++Cl2��+2H2O (2��)
��4����ԭ�ԡ����� (2��) 1.5 mol (2��)
��2��
 (2��)
(2��)MnO2��Cl2��H2O2 (2��)
��3����Ӧ�٣�Cl2+SO2+2H2O= 4H++
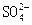 +2Cl�� (2��)
+2Cl�� (2��)��Ӧ�ޣ� MnO2+4H++2Cl��
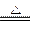 Mn2++Cl2��+2H2O (2��)
Mn2++Cl2��+2H2O (2��)��4����ԭ�ԡ����� (2��) 1.5 mol (2��)
��

��ϰ��ϵ�д�
�����Ŀ
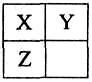
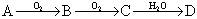 ;��֪DΪǿ�ᣬ��ش�
;��֪DΪǿ�ᣬ��ش� ����ɫ���壬B���д̼�����ζ����ɫ���塣
����ɫ���壬B���д̼�����ζ����ɫ���塣