��Ŀ����
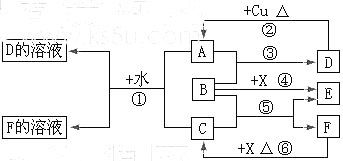
��10�֣�ij�ǽ�������A������ͼ��ʾ�Ĺ���ת��Ϊ������D��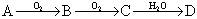 ;��֪DΪǿ�ᣬ��ش�
;��֪DΪǿ�ᣬ��ش�
��1����A������Ϊ ����ɫ���壬B���д̼�����ζ����ɫ���塣
����ɫ���壬B���д̼�����ζ����ɫ���塣
�ٻ���A��ԭ�ӽṹʾ��ͼ_______________________
�ڰ�Bͨ��BaCl2��Һ�У�Ȼ��μ�����H2O2��Һ���а�ɫ�������ɣ��˰�ɫ������ѧʽΪ��
��D��Ũ��Һ�ڼ��ȵ������¿���Cu��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ��
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��д��A���ʵĵ���ʽ ��
��C��D�ķ�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ������������������
��д��A��H2��Ӧ����Ľṹʽ��
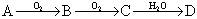 ;��֪DΪǿ�ᣬ��ش�
;��֪DΪǿ�ᣬ��ش���1����A������Ϊ
 ����ɫ���壬B���д̼�����ζ����ɫ���塣
����ɫ���壬B���д̼�����ζ����ɫ���塣�ٻ���A��ԭ�ӽṹʾ��ͼ_______________________
�ڰ�Bͨ��BaCl2��Һ�У�Ȼ��μ�����H2O2��Һ���а�ɫ�������ɣ��˰�ɫ������ѧʽΪ��
��D��Ũ��Һ�ڼ��ȵ������¿���Cu��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ��
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��д��A���ʵĵ���ʽ ��
��C��D�ķ�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ������������������
��д��A��H2��Ӧ����Ľṹʽ��
��10�֣���1���� ��1�֣� �� BaSO4��2�֣�
��Cu+2H2SO4 ==CuSO4+SO2��+2H2O ��2�֣�
��2�����ԣ�1�֣��� 1 ��2��2�֣���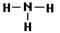 ��2�֣�
��2�֣�
��Cu+2H2SO4 ==CuSO4+SO2��+2H2O ��2�֣�
��2�����ԣ�1�֣��� 1 ��2��2�֣���
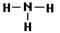 ��2�֣�
��2�֣���

��ϰ��ϵ�д�
�����Ŀ
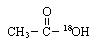 ��Ӧ�Ļ�ѧ����ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��

 �õ����������֮����____________________________��
�õ����������֮����____________________________��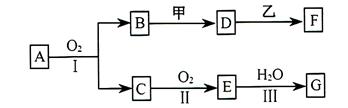
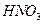 �ữ��
�ữ��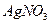 ��Һ�а�ɫ�������ɡ���
��Һ�а�ɫ�������ɡ���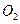 ��Ӧ������̬��B��Cʱ�ų�22.67kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��___________________________________��
��Ӧ������̬��B��Cʱ�ų�22.67kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��___________________________________��