��Ŀ����
��12�֣���֪��A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��E��һ�־�����ζ��Һ�������������������л���֮���ת����ϵ��

��1��A��E�Ľṹ��ʽ�ֱ�Ϊ__________________��____________________��
��2��A��B�ķ�Ӧ����Ϊ________________________��
��3��C�����Ĺ���������_______________�����������õ��Լ�Ϊ____________��
��4��д��B��C�Ļ�ѧ��Ӧ����ʽ��________________________��
��5����ʵ��������Ҳ������ͼ��ʾ��װ����ȡ�������������б���̼������Һ����Ҫ������_______________________________ ��

��6��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�_____________________________ ��
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ����__
_____��

��1��A��E�Ľṹ��ʽ�ֱ�Ϊ__________________��____________________��
��2��A��B�ķ�Ӧ����Ϊ________________________��
��3��C�����Ĺ���������_______________�����������õ��Լ�Ϊ____________��
��4��д��B��C�Ļ�ѧ��Ӧ����ʽ��________________________��
��5����ʵ��������Ҳ������ͼ��ʾ��װ����ȡ�������������б���̼������Һ����Ҫ������_______________________________ ��

��6��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�_____________________________ ��
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ����__
_____��
��1��CH2=CH2 CH3COOCH2CH3 ��2���ӳɷ�Ӧ
��3��ȩ����������Һ������������ͭ����Һ
��4��2CH3CH2OH+O2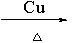 2CH3CHO+2H2O
2CH3CHO+2H2O
��5���кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��6����������7����ֹ���Թ���Һ�屩�С�
��3��ȩ����������Һ������������ͭ����Һ
��4��2CH3CH2OH+O2
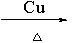 2CH3CHO+2H2O
2CH3CHO+2H2O��5���кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��6����������7����ֹ���Թ���Һ�屩�С�
���������֪A����ϩ����ϩ����̼̼˫��������B�����ʿ�֪����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�������B���Ҵ�����C����ȩ����ȩ�����������ᣬ������Ҵ�����������Ӧ��������������
��3����ȩ�к���ȩ����ȩ���ܷ���������Ӧ������Ƶ�������ͭ����Һ��Ӧ���ݴ˿��Լ���
��5�����ɵ����������к���������Ҵ������ñ���̼������Һ��ȥ�����������кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��6���Ҵ��������ˮ���ǻ��ܵģ����Ե��ܿ�ֱ�Ӳ���ˮ�У���������Һ�嵹����
��7�����ڷ�Ӧ��Ҫ���ȣ����Լ������Ƭ�ܷ�ֹ���Թ���Һ�屩�С�
��3����ȩ�к���ȩ����ȩ���ܷ���������Ӧ������Ƶ�������ͭ����Һ��Ӧ���ݴ˿��Լ���
��5�����ɵ����������к���������Ҵ������ñ���̼������Һ��ȥ�����������кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��6���Ҵ��������ˮ���ǻ��ܵģ����Ե��ܿ�ֱ�Ӳ���ˮ�У���������Һ�嵹����
��7�����ڷ�Ӧ��Ҫ���ȣ����Լ������Ƭ�ܷ�ֹ���Թ���Һ�屩�С�

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ

