��Ŀ����
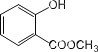
����Ŀ��![]() ��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã�
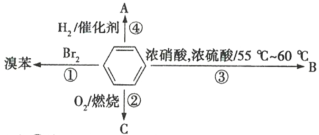
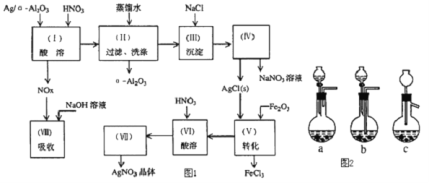
��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã�![]() �������Ҳ��������ᣬ�ô����Ļ���ʵ����ͼ1��ʾ�����е�ת����ӦΪ��
�������Ҳ��������ᣬ�ô����Ļ���ʵ����ͼ1��ʾ�����е�ת����ӦΪ��![]()
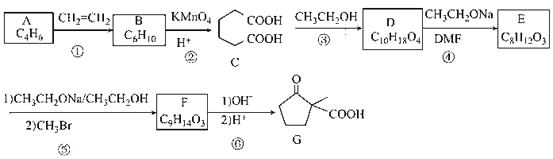
�Ķ�����ʵ�����̣����������գ�
![]() �����ܽ�Ӧ��ѡ��װ��
�����ܽ�Ӧ��ѡ��װ��![]() ͼ
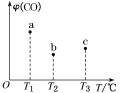
ͼ![]() ______
______ ![]() ѡ��a��b��
ѡ��a��b��![]() ��
��
![]() ��ʵ�����
��ʵ�����![]() ��
��![]() �����������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽ ______ ��
�����������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽ ______ ��
![]() ʵ�����
ʵ�����![]() ��
��![]() ���貣������Ϊ ______
���貣������Ϊ ______ ![]() ��д����
�����![]() ��
��
![]() ʵ�����
ʵ�����![]() ��
��![]() ��
��![]() ��Һ���
��Һ���![]() ������Ҫ���е�ʵ���������Ϊ�� ______
������Ҫ���е�ʵ���������Ϊ�� ______ ![]() ��ѡ�۷�
��ѡ�۷�![]() ��
��
![]() ����
����![]() ����
����![]() ����
����![]() ��ȴ�ᾧ
��ȴ�ᾧ
![]() ��֪��
��֪��![]() ��
��
![]()
NO��![]() �Ļ���������ɿɱ�ʾΪ
�Ļ���������ɿɱ�ʾΪ![]() �û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ ______
�û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ ______
![]()
![]()
![]()
![]() ��֪
��֪![]() ��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ ______ �� ______ ��
��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ ______ �� ______ ��
���𰸡�![]()
![]() ©�����ձ���������
©�����ձ��������� ![]()
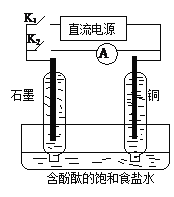
![]() ����������
���������� ![]() ������
������
��������
������-Al2O3������ϡ���ᣬAg/a-Al2O3�м���ϡ��������ܽ����������������˺���Һ�к���AgNO3������Ϊ��-Al2O3����Һ�м���NaCl�õ�����ΪAgCl���ٹ��˷���õ�AgNO3��NaNO3��Һ��AgClת��ΪAg2O���ܽ���˷��룬���������ܽ�Ag2O�õ�AgNO3������Ũ������ȴ�ᾧ�����˵ȵõ�AgNO3���壻
(1)��Ӧ���ɵ������������壬��Ҫ����������������Һ���գ�װ��Ӧ�ܱգ������ܲ�����Һ�����£�
(2)����ˮ�к���������������ˮ��Ӧ����HCl��HClO��HCl��AgNO3��Ӧ����AgCl��HNO3��
(3)ʵ�����(��)Ϊ����������Һ��ӦΪ���ˣ��ݴ���д���貣��������
(4)����Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����˵Ȳ�����
(5)���ݷ�Ӧ��NO+NO2+2NaOH�T2NaNO2+H2O��2NO2+2NaOH�TNaNO2+NaNO3+H2O���������ͨ��NaOH��Һ����ȫ����ʱ��Ӧ����n(NO2)��n(NO)���ɣ�
(6)��֪���������������������������Ļ����ʣ�������֪����������������������������
����![]() ������ϡ���ᣬ
������ϡ���ᣬ![]() �м���ϡ��������ܽ����������������˺���Һ�к���
�м���ϡ��������ܽ����������������˺���Һ�к���![]() ������Ϊ
������Ϊ![]() ����Һ�м���NaCl�õ�����ΪAgCl���ٹ��˷���õ�
����Һ�м���NaCl�õ�����ΪAgCl���ٹ��˷���õ�![]() ��
��![]() ��Һ��AgClת��Ϊ
��Һ��AgClת��Ϊ![]() ���ܽ���˷��룬���������ܽ�
���ܽ���˷��룬���������ܽ�![]() �õ�
�õ�![]() ������Ũ������ȴ�ᾧ�����˵ȵõ�
������Ũ������ȴ�ᾧ�����˵ȵõ�![]() ���壻
���壻
![]() ��Ӧ���ɵ������������壬��Ҫ����������������Һ���գ�װ��b�в��������岻�ܵ������ᵼ��װ����ѹǿ��ǿ������ը�ѵ�Σ�գ�cװ�ò��ܱգ���ѡa��
��Ӧ���ɵ������������壬��Ҫ����������������Һ���գ�װ��b�в��������岻�ܵ������ᵼ��װ����ѹǿ��ǿ������ը�ѵ�Σ�գ�cװ�ò��ܱգ���ѡa��
![]() ����ˮ�к���������������ˮ��Ӧ����HCl��HClO��HCl��
����ˮ�к���������������ˮ��Ӧ����HCl��HClO��HCl��![]() ��Ӧ����AgCl��
��Ӧ����AgCl��![]() ����Ӧ���ӷ���ʽΪ��
����Ӧ���ӷ���ʽΪ����
![]() ʵ�����
ʵ�����![]() ��
��![]() Ϊ����������Һ��ӦΪ���ˣ������貣������Ϊ©�����ձ�����������
Ϊ����������Һ��ӦΪ���ˣ������貣������Ϊ©�����ձ�����������
![]() ��
��![]() ��Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����˵Ȳ�����˳��Ϊbd���ʴ�Ϊ��bd��
��Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����˵Ȳ�����˳��Ϊbd���ʴ�Ϊ��bd��
![]() �ɷ���ʽ��֪�������ͨ��NaOH��Һ����ȫ����ʱ��Ӧ����
�ɷ���ʽ��֪�������ͨ��NaOH��Һ����ȫ����ʱ��Ӧ����![]() ����
����![]() ����ѡ��c��
����ѡ��c��
![]() Ҫ����Ag�Ļ����ʱ���֪����������������������Ag�������������Ag������������Ҫ֪���������
Ҫ����Ag�Ļ����ʱ���֪����������������������Ag�������������Ag������������Ҫ֪���������![]() ��������
��������