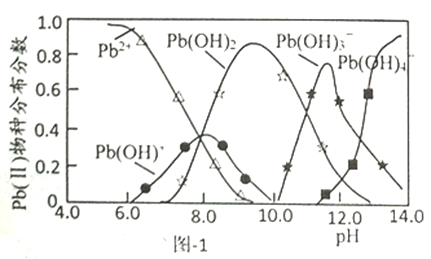
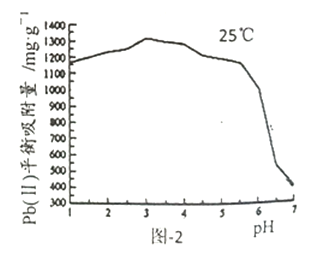
��Ŀ����
����Ŀ�����ݻ�Ϊ10 L���ܱ������г���1mol H2��1mol I2,����һ���¶���ʹ�䷢����Ӧ:H2(g)+I2(g) ![]() 2HI(g)��20min��ﵽƽ��,���c(HI)=0.04 mol/L��
2HI(g)��20min��ﵽƽ��,���c(HI)=0.04 mol/L��
(1)��Ӧ�ӿ�ʼ��ƽ��ʱ��v(H2)Ϊ_______,ƽ�ⳣ��K=__________��
(2)�¶Ȳ��䣬�ﵽƽ������������ٳ���1molHI����,ƽ����________ (������������ƶ���)�ƶ�;�ٴ�ƽ���,HI�����������________ (���������С�����䡱)
(3)��(2)����ƽ����ϵ����,ƽ���I2���������Ϊ45%,�����º�H2��ת���ʽ�_____(���������С���� �䡱)���÷�Ӧ�Ħ�H_____0(�>������ <����=��)��
(4)���(3)�뻭��2HI(g)![]() H2(g)+I2(g)��ƽ�ⳣ��K���¶ȱ仯������ͼ��
H2(g)+I2(g)��ƽ�ⳣ��K���¶ȱ仯������ͼ��
 _____
_____
���𰸡� 0.001mol/(L��min) 0.25 ���� ���� ��С < 
��������H2(g)+I2(g)![]() 2HI(g)
2HI(g)
��ʼŨ�ȣ�mol/L�� 0.1 0.1 0
ת��Ũ�ȣ�mol/L�� 0.02 0.02 0.04
ƽ��Ũ�ȣ�mol/L�� 0.08 0.08 0.04
��1����Ӧ�ӿ�ʼ��ƽ��ʱ��v(H2)��0.02mol/L��20min��0.001mol/(L��min)��ƽ�ⳣ��K=![]() ����2�����ڷ�Ӧǰ��������䣬����¶Ȳ��䣬�ﵽƽ������������ٳ���1molHI���壬�൱������ѹǿ��ƽ�ⲻ�ƶ��������ٴ�ƽ�����HI�����������������3������2������ƽ����ϵ������ƽ���I2���������Ϊ45%���������¶�֮ǰI2���������Ϊ
����2�����ڷ�Ӧǰ��������䣬����¶Ȳ��䣬�ﵽƽ������������ٳ���1molHI���壬�൱������ѹǿ��ƽ�ⲻ�ƶ��������ٴ�ƽ�����HI�����������������3������2������ƽ����ϵ������ƽ���I2���������Ϊ45%���������¶�֮ǰI2���������Ϊ![]() ����˵�������¶�ƽ�����淴Ӧ������У��������º�H2��ת���ʽ���С���÷�Ӧ����H��0����4������Ӧ�Ƿ��ȷ�Ӧ���������¶�ƽ�����淴Ӧ������У�ƽ�ⳣ����С����˷�Ӧ2HI(g)
����˵�������¶�ƽ�����淴Ӧ������У��������º�H2��ת���ʽ���С���÷�Ӧ����H��0����4������Ӧ�Ƿ��ȷ�Ӧ���������¶�ƽ�����淴Ӧ������У�ƽ�ⳣ����С����˷�Ӧ2HI(g)![]() H2(g)+I2(g)��ƽ�ⳣ��K���¶ȵ����߶�������仯������ͼΪ
H2(g)+I2(g)��ƽ�ⳣ��K���¶ȵ����߶�������仯������ͼΪ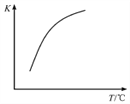 ��
��

 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�