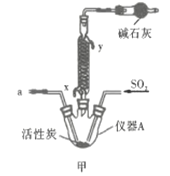
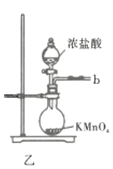
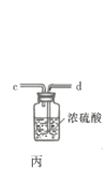
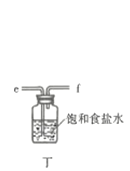
��Ŀ����
����Ŀ���Ȼ��ƣ�NaCl���������г��õĻ�ѧƷ��Ҳ����Ҫ�Ļ�������ԭ�ϡ�
��1��ijͬѧ������Ԫ�صIJ�ͬ��������Ʊ�NaCl�������о�3�ַ�Ӧ��
��2Na+Cl2![]() 2NaCl
2NaCl
��___��
��___��
��___��
��2��������һ�㺬��CaCl2��MgCl2��CaSO4��MgSO4���ʡ��Ӵ����Ƶþ��εĹ������£�

��д��MgCl2��CaSO4�ĵ��뷽��ʽ��___��___��
�ڸ������ֱ��ǣ�����a___������b___������c___��
���Լ�����___��
�ܼ����Լ��������������ӷ���ʽΪ___�������Լ��������������ӷ���ʽΪ___��
���𰸡�Na2O+2HCl=2NaCl+H2O NaOH+HCl=NaCl+H2O Na2CO3+2HCl=2NaCl+H2O+CO2�� MgCl2=Mg2++2Cl- CaSO4=Ca2++SO42- ���� ���� �����ᾧ��Һ BaCl2 CO32-+Ba2+=BaCO3����CO32-+Ca2+=CaCO3�� CO32-+2H+=H2O+CO2����OH-+H+=H2O
��������
��1����Na2O�����ᷴӦ�������Ȼ��ƣ�
������кͷ�Ӧ�ܹ������Ȼ��ƣ�
�ܴ��������ᷴӦ�������Ȼ��ƣ�
��2�������д��ڲ�������ɳ�Ϳ����Ե�CaCl2��MgCl2��CaSO4��MgSO4�����ʣ����˿ɳ�ȥ��������ɳ������BaCl2��NaOH��Na2CO3�ɽ�����һһ��ȥ���ݴ˻ش����⡣
��1����Na2O�����ᷴӦ�������Ȼ��ƣ���ӦΪ��Na2O+2HCl=2NaCl+H2O��
������кͷ�Ӧ�ܹ������Ȼ��ƣ���ӦΪ��NaOH+HCl=NaCl+H2O
�ܴ��������ᷴӦ�������Ȼ��ƣ���ӦΪ��Na2CO3+2HCl=2NaCl+H2O+CO2����
��2�������д��ڲ�������ɳ�Ϳ����Ե�CaCl2��MgCl2��CaSO4��MgSO4�����ʣ����˿ɳ�ȥ��������ɳ��������aΪ���ˣ����õ���ҺA�д���CaCl2��MgCl2��CaSO4��MgSO4�����ʣ����������BaCl2��Һ���ɳ�ȥSO42-�����������NaOH��Һ��ȥ���е�Mg2+�����������Na2CO3�ɳ�ȥ��Һ�����е�Ba2+�������г���һ���Թ��˺����ҺB����ô����bΪ���ˣ��Լ�����BaCl2��Һ���Լ�����Na2CO3��Һ����ҺB�д���NaCl������Na2CO3��Һ��������������Ტ���ȣ��ɳ�ȥ������Na2CO3��Һ�����յõ�������NaCl��Һ�����Լ���Ϊ���ᣬ����cΪ�����ᾧ��
��MgCl2�ĵ��뷽��ʽΪ��MgCl2=Mg2++2Cl-��CaSO4�ĵ��뷽��ʽΪ��CaSO4=Ca2++SO42-��
���ɷ�����֪������a�ǹ��ˣ�����b�ǹ��ˣ�����c�������ᾧ��
���ɷ�����֪���Լ�����BaCl2��Һ��
�ܼ����Լ��������������ӷ���ʽΪCO32-+Ca2+=CaCO3����CO32-+Ba2+=BaCO3���������Լ��������������ӷ���ʽΪCO32-+2H+=H2O+CO2����OH-+H+=H2O��

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�