��Ŀ����
����Ŀ������������Ϊ���Ͻ��ά���ء��������ڴ��������͵�ء���(V)����Һ����Ҫ��VO43-(��ɫ)��VO2+(dz��ɫ)��VO2+(��ɫ)��V3+(��ɫ)��V2+(��ɫ)����ʽ���ڡ��ش��������⣺
(1)��ҵ������V2O5�Ʊ���������
4Al(s)+3O2(g)=2Al2O3(s) ��H1=-2834kJ/mol
4V(s)+5O2(g)=2V2O5(s) ��H2=-3109kJ/mol
д��V2O5��A1��Ӧ�Ʊ����������Ȼ�ѧ����ʽ____________________��
(2)V2O5��һ����Ҫ������������������ʣ�
��V2O5��ǿ������Һ����VO43-��ʽ���ڣ���д��V2O5����NaOH��Һ�����ӷ���ʽ��___________________________________________��
��V2O5����ǿ�����ԣ�����Ũ������Եõ���ɫ��Һ����д��V2O5��Ũ���ᷴӦ�Ļ�ѧ��Ӧ����ʽ��________________________________��

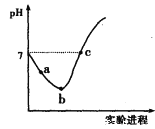
(3)VO43-��V2O74-��PH��13����Һ�п��ת���������£�1.0 mol��L-1��Na3VO4��Һ��c(VO43-)��c(H+)�ı仯��ͼ��ʾ��
��д����Һ��Na3VO4ת��ΪNa4V2O7�����ӷ���ʽ__________________��
����ͼ��֪����Һ��c(H+)����VO43-��ƽ��ת����______(�������С�����䡱)������A�����ݣ������ת����Ӧ��ƽ�ⳣ������ֵΪ_____________��
(4)ȫ��Һ�������һ��������������索���豸���乤��ԭ����ͼ��ʾ��

�ٷŵ������A�缫�ķ�ӦʽΪ__________________��
�ڳ������У�B�缫������Һ��ɫ�仯Ϊ___________________��
���𰸡� 10Al(s)+3V2O3��s��=5Al2O3(s)+6V(s) ��H=-2421.5kJ/mol V2O5+6OH-=2VO43-+3H2O V2O5+6HCl=2VOCl2+Cl2��+3H2O 2VO43-+H2O![]() V2O74-+2OH- ���� 0.4 VO2++2H++e-=VO2++H2O ��Һ����ɫ��Ϊ��ɫ
V2O74-+2OH- ���� 0.4 VO2++2H++e-=VO2++H2O ��Һ����ɫ��Ϊ��ɫ
����������1����4Al(s)+3O2(g)=2Al2O3(s) ��H1=-2834kJ/mol
��4V(s)+5O2(g)=2V2O5(s) ��H2=-3109kJ/mol
���ݸ�˹�����ɢ١�2.5-�ڡ�1.5�ɵ÷�Ӧ10Al(s)+3V2O3��s��=5Al2O3(s)+6V(s) ��H=2.5��H1-1.5��H2=2.5����-2834kJ/mol��-1.5����-3109kJ/mol��=-2421.5kJ/mol��
(2) �� V2O5����NaOH��Һ����VO43-���䷴Ӧ�����ӷ���ʽΪ��V2O5+6OH-=2VO43-+3H2O��
��V2O5����ǿ�����ԣ�����Ũ���Ὣ����������������ͬʱ�õ���ɫ��Һ����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��V2O5+6HCl=2VOCl2+Cl2��+3H2O��
��3������ͼ��֪����PH��13����Һ�У� VO43-���ˮ�������������ת��ΪV2O74-�����ӷ���ʽΪ��2VO43-+H2O![]() V2O74-+2OH-������ͼ��֪����Һ��c(H+)����VO43-��ƽ��ת����������A�����ݣ�c(VO43-)=0.2mol/L���ı��c(VO43-)=1.0mol/L-0.2mol/L=0.8mol/L����c(V2O74-)=
V2O74-+2OH-������ͼ��֪����Һ��c(H+)����VO43-��ƽ��ת����������A�����ݣ�c(VO43-)=0.2mol/L���ı��c(VO43-)=1.0mol/L-0.2mol/L=0.8mol/L����c(V2O74-)=![]() ��0.8mol/L =0.4mol/L��c(H+)=5��10-14 mol/L��c(OH-)=0.2mol/L����ת����Ӧ��ƽ�ⳣ������ֵΪ��
��0.8mol/L =0.4mol/L��c(H+)=5��10-14 mol/L��c(OH-)=0.2mol/L����ת����Ӧ��ƽ�ⳣ������ֵΪ�� 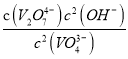 =
=![]() =0.4mol/L����4���ٷŵ��������Ϊԭ��ص�����A�缫VO2+�õ��Ӳ���VO2+���䷴ӦʽΪ��VO2++2H++e-=VO2++H2O���ڳ���������Ϊ���أ�����B�缫����V3+�õ���ת��ΪV2+����Һ����ɫ��Ϊ��ɫ��
=0.4mol/L����4���ٷŵ��������Ϊԭ��ص�����A�缫VO2+�õ��Ӳ���VO2+���䷴ӦʽΪ��VO2++2H++e-=VO2++H2O���ڳ���������Ϊ���أ�����B�缫����V3+�õ���ת��ΪV2+����Һ����ɫ��Ϊ��ɫ��

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�����Ŀ�����и�������֮�䲻����ʵ����ͼ��ʾת�����ǣ� ��
![]()
ѡ�� | X | Y | Z | M |
A | S | SO2 | SO3 | O2 |
B | HNO3 | Fe(NO3)3 | Fe(NO3)2 | Fe |
C | NaOH | Na2CO3 | Na2CO3 | CO2 |
D | HN3 | NO | NO2 | O2 |
A. A B. B C. C D. D