��Ŀ����
����Ŀ�����ܱ������У���һ����A��B�����Ϻ�����Ӧ��xA(g)��yB(g)![]() zC(g)��mD(s)��ƽ��ʱ���A��Ũ��Ϊ0.5mol/L�������¶Ȳ��䣬���������ݻ�����ԭ�����������ٴ�ƽ��ʱ�����A��Ũ��Ϊ0.3mol/L���������й��ж���ȷ���� �� ��
zC(g)��mD(s)��ƽ��ʱ���A��Ũ��Ϊ0.5mol/L�������¶Ȳ��䣬���������ݻ�����ԭ�����������ٴ�ƽ��ʱ�����A��Ũ��Ϊ0.3mol/L���������й��ж���ȷ���� �� ��
A. x��y��z��m B. ���������ܶ�һ����С
C. ƽ��������Ӧ�����ƶ� D. B��ת����һ����С
���𰸡�D
��������ƽ��ʱA��Ũ��Ϊ0.5mol/L�������¶Ȳ��䣬���������ݻ�����ԭ��������������ƽ�ⲻ�ƶ�����A��Ũ��Ӧ����0.25mol/L���ٴ�ƽ��ʱ�����A��Ũ��Ϊ0.3mol/L��˵��ƽ�������ƶ�������D�ǹ��壬�����Ƴ�x��y��z��ƽ�������ƶ���������������������������ܶȲ�һ����С��ƽ�������ƶ���B��ת����һ����С����ѡD��

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�����Ŀ�������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
����[��������]
��1��Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
��2����Na2CO3��Na2S���Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ�г���������Na2SO3��Na2SO4��
��3��Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��4�������������ⷴӦ�����ӷ���ʽΪ��2S2O32-+I2=S4O62-+2I-
����[�Ʊ���Ʒ]ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)
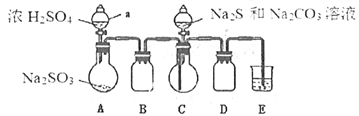
ʵ�鲽�裺
��1������ͼ��ʾ��װ��װ�ú�Ӧ��_______________(���������)���ٰ�ͼʾ�����Լ�������B��D��������____________________________��E�е��Լ���__________(ѡ��������ĸ���)��
A��ϡH2SO4 B��NaOH��Һ C������NaHSO3��Һ
��2��������ƿC�м���Na2S��Na2CO3�����Һ��������ƿA�еμ�ŨH2SO4��C�з�Ӧ����Na2S2O3��CO2����ѧ����ʽΪ______________________��
��3����Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л��Һ����Һ���������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
����[̽���뷴˼]
��1��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��)��
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�____________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
��2����I2�ı���Һ�ⶨ��Ʒ�Ĵ���
ȡ10.0g��Ʒ�����Ƴ�100mL��Һ��������Һ������ˮ���뾭����С���ȴ�����ʹ�ã���Ŀ����ɱ������__________��������̼��ȡ10.00mL��Һ����________��ҺΪָʾ������Ũ��Ϊ0.10mol/LI2�ı��ܲ����еζ���������ݼ�¼���±���ʾ��
��� | 1 | 2 | 3 |
��Һ�����/mL | 10.00 | 10.00 | 10.00 |
����I2����Һ�����/mL | 19.95 | 17.10 | 20.05 |
�ζ�ʱ���ﵽ�ζ��յ��������___________________________________________��Na2S2O3��5H2O�ڲ�Ʒ�е�����������_______________(�ðٷ�����ʾ���ұ���1λС��)��