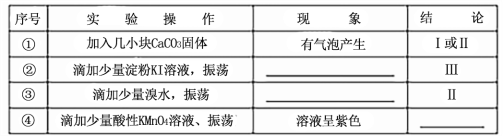
��Ŀ����
����Ŀ��ij��ѧ��ȤС�����ʵ�飬�ⶨNa2CO3��NaHCO3�������Na2CO3������������
������
ʵ�鲽��Ϊ���ٳ���������������ΪA g���ڳ���װ����������������ΪB g���ۼ��ȣ�����ȴ���ݳ��������Ͳ����������ΪC g�����ظ������ݲ�����ֱ�����أ�����ΪD g��
��1�������з�����Ӧ�Ļ�ѧ����ʽ ��
��2������Na2CO3�������������õ��IJⶨ����Ϊ ��
�ҷ�����
����ƽ��ȷ��ȡ 0.3000g��Ʒ��������ƿ�У���������ˮ�ܽ⣬����2�η�̪��Һ����0.1000mol��L-1�ı�����ζ�����Һ�ɷۺ�ɫ�պñ�Ϊ��ɫ���ﵽ�ζ��յ�ʱ����ΪNaHCO3����HCl+Na2CO3 == NaHCO3+NaCl���ظ������������Ρ�
��3�����������������Һ100ml������2.0mol/LHCl�������ƣ�������Ͳ��ȡ��HCl��Һ ml������ʱ���ӿ̶��ߣ����������Ƶ���ҺŨ�� ��������ƫ��������ƫ��������û��Ӱ������
��4�������±����ݣ���Ʒ��w(Na2CO3)= �����ðٷ�����ʾ������һλС����
�ζ����� | ��Ʒ������/g | ϡ��������/mL | |
�ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
1 | 0.3000 | 1.02 | 21.03 |
2 | 0.3000 | 2.00 | 21.99 |
3 | 0.3000 | 0.20 | 20.20 |
��������
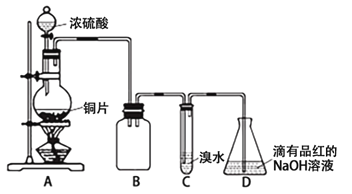
��ȡm g��Ʒ��ѡ����ͼ����װ�òⶨ��Ʒ�����ᷴӦ���ɵ����������
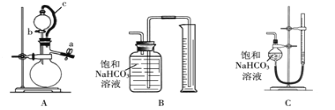
��5��ijͬѧ�����·������װ��A�������ԣ��ڷ�Һ©���м�������ˮ����ͼ���Ӻ�װ�ã��ر�ֹˮ��a����ֹˮ�м�ס��Ƥ��c������b������Һ©���е�ˮ���������£����ж�װ��A�Ƿ�©���� ���©����������©��������ȷ��������ʵ��ʱ��װ��A��c������ �� ��
��6��Ϊ����߲ⶨ��ȷ�ԣ�Ӧѡ��װ��A�� ����д��ĸ��ţ�����ʵ�顣
���𰸡���1��2NaHCO3![]() Na2CO3+CO2�� +H2O ����
Na2CO3+CO2�� +H2O ����
��2��A��B��D ����3��5.0��ƫ�ߣ���4��70.7% ��
��5����ȷ����ƽ����ѹ��������Һ����������С����Һ����������������� ����6��C ��
��������
�����������1��̼�����Ʋ��ȶ������ֽ⣬�����ڷ�����Ӧ�ǣ�2NaHCO3![]() Na2CO3+CO2�� +H2O ������2���˷������Ȳ���NaHCO3��������Ȼ������������ȥ̼�����Ƶ��������õ�̼���Ƶ��������Ӷ����̼���Ƶ����������������ҪA��B����̼�����Ƶ��������ò�������⣬�����ҪD��������Cֻ��˵��̼�������Ƿ���ȫ�ֽ⣬����Ҫ��(3)��Ͳ�ľ�ȷ����0.1mL��ϡ��ǰ���������ʵ������䣬��100��10��3��0.1000=V(HCl)��10��3��2�����V(HCl)=5.0mL������ʱ�����Ӷ�����������Һ��������٣�Ũ��ƫ�ߣ�(4)�����������������ֱ���20.01��19.99��20.00��ƽ�������������Ϊ(20.01+19.99+20.00)/3mL=20.00mL�����ݷ�Ӧ����ʽ��n(Na2CO3)=20.00��10��3��0.1000mol=2��10��3mol��m(Na2CO3)=2��10��3��106g=0.212g��̼���Ƶ���������Ϊ0.212/0.3000��100%=70.7%��(5)c��������ʹ��Һ©���е�ѹǿ����ƿ��ѹǿ��ȣ��ܹ�ʹҺ��˳�����£������ȷ���Ƿ�©����ƽ����ѹ��������Һ����������С����Һ����������������� ��(6)ͨ����ˮ����������������Bװ���е��ܺ���ˮ���ⲿ��ˮ�������ų��ģ�����ȡ��������Cװ�ÿ��Լ�����
Na2CO3+CO2�� +H2O ������2���˷������Ȳ���NaHCO3��������Ȼ������������ȥ̼�����Ƶ��������õ�̼���Ƶ��������Ӷ����̼���Ƶ����������������ҪA��B����̼�����Ƶ��������ò�������⣬�����ҪD��������Cֻ��˵��̼�������Ƿ���ȫ�ֽ⣬����Ҫ��(3)��Ͳ�ľ�ȷ����0.1mL��ϡ��ǰ���������ʵ������䣬��100��10��3��0.1000=V(HCl)��10��3��2�����V(HCl)=5.0mL������ʱ�����Ӷ�����������Һ��������٣�Ũ��ƫ�ߣ�(4)�����������������ֱ���20.01��19.99��20.00��ƽ�������������Ϊ(20.01+19.99+20.00)/3mL=20.00mL�����ݷ�Ӧ����ʽ��n(Na2CO3)=20.00��10��3��0.1000mol=2��10��3mol��m(Na2CO3)=2��10��3��106g=0.212g��̼���Ƶ���������Ϊ0.212/0.3000��100%=70.7%��(5)c��������ʹ��Һ©���е�ѹǿ����ƿ��ѹǿ��ȣ��ܹ�ʹҺ��˳�����£������ȷ���Ƿ�©����ƽ����ѹ��������Һ����������С����Һ����������������� ��(6)ͨ����ˮ����������������Bװ���е��ܺ���ˮ���ⲿ��ˮ�������ų��ģ�����ȡ��������Cװ�ÿ��Լ�����

 ����5��2���ϵ�д�
����5��2���ϵ�д�