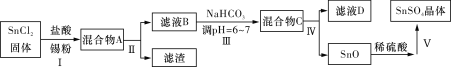
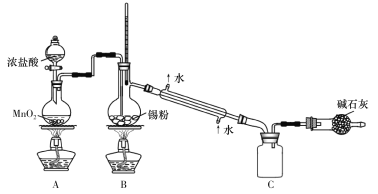
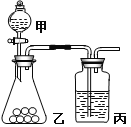
��Ŀ����
����Ŀ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ�����У���������������������������ȣ���ش��������⣺
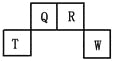
��1��W�����ڱ��е�λ���� ��Q��R��T����Ԫ��ԭ�Ӱ뾶�ɴ�С˳��Ϊ ����Ԫ�ط��ű�ʾ����Q�����������ĵ���ʽΪ ��R�⻯����ӵĽṹʽΪ ��
��2��Ԫ�ص�ԭ�ӵõ���������Q W������ǿ������������������
��3��ԭ�������ȣҶ�1��Ԫ����һ���⻯���ֽܷ�Ϊ������һ���⻯��÷�Ӧ�Ļ�ѧ����ʽΪ ��
���𰸡���1����������VIA�壬Al��C��N��![]() ��
�� ����2�����ڣ���3��2H2O2
����2�����ڣ���3��2H2O2![]() 2H2O+O2����
2H2O+O2����
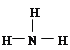
�������������������1����Ϊ�Ƕ����ڣ��������ڱ��Ľṹ��T��������������������������ȣ���TΪAl���Ƴ�Q��C��R��N��W��S��Sλ�ڵ�������VIA�壬�뾶һ�����Ӳ��������Ӳ���Խ�࣬�뾶Խ����ԭ�����������Ӳ�����ȣ��뾶��ԭ�������ĵ�������С�������Al��C��N��Q�����������ΪCO2���������Ϊ![]() ��R���⻯��ΪNH3����ṹʽΪ��
��R���⻯��ΪNH3����ṹʽΪ�� ����2��S�ĵõ�������ǿ��C����3����R��1��Ԫ����O������һ���⻯���ֽܷ����һ���⻯����⻯��ΪH2O2���䷴Ӧ����ʽΪ��2H2O2
����2��S�ĵõ�������ǿ��C����3����R��1��Ԫ����O������һ���⻯���ֽܷ����һ���⻯����⻯��ΪH2O2���䷴Ӧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����