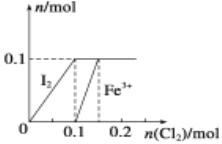
��Ŀ����
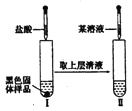
����Ŀ��ijͬѧ��ʵ���Ҵ���ͼ��I����ǩ���Լ�ƿ��ȡ�����ƽ���ȼ��ʵ�飬��ͼ��II����
��1��װ�õ�����Ϊ____________��ʵ���У��۲쵽�ĵ���ɫ�Ĺ���������____________��д��ѧʽ�����۲쵽�Ļ�ɫ������___________Ԫ�صķ�����ס�
��2��ʵ����ֻ���������ɫ�������ɡ��ӷ�Ӧ�Pʵ������²⣺�ú�ɫ���ʿ���Ϊ̼����һ����������ɵĻ���
��������������___________��____________(д��ѧʽ����
��3���Ժ�ɫ�������ʵ��������ͼ��ʾ̽����
��ʵ��I�м��������Ŀ���ǣ�________��
����ͨ��ʵ��II�������IJ����ܿ���ȷ����ɫ���ʵ���ɣ������Ƹ���ơ�
����ѡ�Լ���ϡ���ᡢKSCN��Ũ��K3[Fe(CN)6] ��Һ��l0����H2O2��Һ��
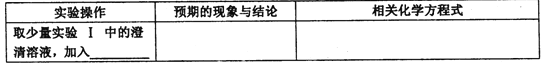
���𰸡���1��������Na2O2���ƣ���2��Fe3O4��FeO��
��3���������ɫ�������Ƿ���̼��ͬʱ�ܽ������
��������KSCN��Һ�������Һ�Ժ�ɫ�����ɫ����ΪFe3O4����֮��ΪFeO��Fe3++3SCN-=Fe(SCN)3
��������
�����������1������ͼʾ�����Ĺ����֪������Ϊ������ʵ���У��۲쵽�ĵ���ɫ�Ĺ��������ǹ������ƣ��۲쵽�Ļ�ɫ��������Ԫ�صķ������
��2��ʵ����ֻ���������ɫ�������ɣ�����ͼ1��֪���������л���������������������Ӧ���ɵĺ�ɫ������Fe3O4��FeO���ʸ������������Fe3O4��FeO��
��3�������ں�ɫ�������ʿ���Ϊ̼�����������̼���ʲ��������ᣬ�������������������ᣬ���Լ�������Ŀ���Ǽ����ɫ�����������Ƿ���̼��ͬʱ�ܽ������
��ȡ����ʵ����������Һ������������KSCN��Һ�������Һ�Ժ�ɫ�����ɫ����ΪFe3O4�������Һû�б�ɺ�ɫ��˵����ɫ����ΪFeO����Ӧ�����ӷ���ʽΪFe3++3SCN-=Fe(SCN)3��

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�