��Ŀ����
����Ŀ���ǽ�������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⡣
![]()
![]()
![]()
![]()
![]()
![]()
![]()
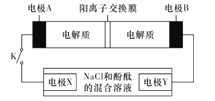
��1����A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ����:
��D�Ļ�ѧʽ��________��
���ڹ�ҵ�����У�B����Ĵ����ŷű���ˮ���պ��γ���________����Ⱦ�˻�����
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ������:
��A��C�Ļ�ѧʽ�ֱ��ǣ�A________��C________��
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬��д���÷�Ӧ�Ļ�ѧ����ʽ_______________________________________________���÷�Ӧ________(����������������������)������ԭ��Ӧ��
���𰸡���ÿ��2�֣���12�֣�
��1����H2SO4������
��2����N2NO2
��Cu��4HNO3(Ũ)===Cu(NO3)2��2NO2����2H2O ����
��������
��1��S��O2��������SO2��SO2�ٱ�O2��������SO3��SO3��ˮ��Ӧ����H2SO4��
��2��N2��O2��������NO��NO�ٱ�O2��������NO2��NO2��ˮ��Ӧ����HNO3

����Ŀ���ס��ҡ���������Ϊ��ѧ��ѧ�����Ĵ��������֮������ͼ��ʾ�ķ�Ӧ��ϵ�����������鲻��������ת����ϵ����( )
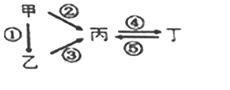
ѡ�� | �� | �� | �� | �� |
A | N2 | NH3 | NO | NO2 |
B | Si | SiO2 | Na2SiO3 | Na2CO3 |
C | S | H2S | SO3 | H2SO4 |
D | Al | Al2O3 | NaAlO2 | Al��OH��3 |
A. AB. BC. CD. D