��Ŀ����
����Ŀ��ij���ε�س䡢�ŵ�Ļ�ѧ����ʽΪ2K2S2+KI3![]() K2S4+3KI�������������豸���ӵĵ�·��ͼ��ʾ�����պϿ���Kʱ���缫X������Һ�ȱ�졣������˵����ȷ����
K2S4+3KI�������������豸���ӵĵ�·��ͼ��ʾ�����պϿ���Kʱ���缫X������Һ�ȱ�졣������˵����ȷ����
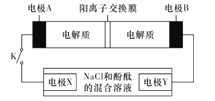
A. �ŵ�ʱ���缫A������ԭ��Ӧ
B. �缫A�ĵ缫��ӦʽΪ3I--2e-=I3-
C. ����0.1mol K+ͨ�������ӽ���Ĥʱ���缫X�ϲ�������1.12 L(��״����)
D. ��س��ʱ���缫BҪ�����Դ�������������缫�Ϸ�����ԭ��Ӧ
���𰸡�C
����������������ͼʾ��Ϣ�ɵã����պϿ���Kʱ��X������Һ�ȱ�죬��˵��X������OH-���ɣ�����X���Ϸ����õ��ӵĻ�ԭ��Ӧ����������X����������Y������������������������ԭ��صĸ��������Ե缫A�Ǹ������缫B��������
A��ŵ�ʱAΪ�����������Ϸ���ʧ���ӵ�������Ӧ����A����B��缫AΪ����������������Ӧ����ϵ���ܷ�Ӧ����缫��ӦʽΪ��2S22--2e-=S42-����B����C�����0.1molK+ͨ�����ӽ���Ĥʱ����·��ת��0.1mol���ӣ�������������ӵĹ�ϵʽH2~2e-���ɼ����������������Ϊ1.12L(��״����)����C��ȷ��D���س��ʱ��������(�缫B)Ҫ�����Դ������������������������������Ӧ����D����

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�����Ŀ��������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJmol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJmol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJmol-1 | K3 |
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g)![]() CH3OCH3(g)��CO2(g) ��H
CH3OCH3(g)��CO2(g) ��H
�÷�Ӧ��H��__________________����ѧƽ�ⳣ��K��____________________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g) ![]() CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч���� D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����_______________________________��
