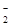��Ŀ����
ij�����Һ�п��ܴ������е��������±���ʾ��
|
������ |
H����K����Al3����NH4+��Mg2�� |
|
������ |
Cl����Br����OH����CO32-��AlO2- |
Ϊ̽����ɷ֣�ijͬѧ��Na2O2���뵽���������Һ�в��ȣ�������������������ʵ��������Na2O2�����ʵ����Ĺ�ϵ�ֱ���ͼ��ʾ��

��1������Һ��һ�����е���������______________����
��Ӧ���ʵ���Ũ��֮��Ϊ________����Һ��һ����
���ڵ���������_____________��
��2����д���������ٵ����ӷ���ʽ_____________________��
��1��H����Al3����NH4+ ��Mg2����2�֣���2:2:2:3��2�֣���OH����CO32-��AlO2-��2�֣�
��2��Al(OH)3+OH��= AlO2- +2H2O��2�֣�
��������
�����������1������0��amol��������ʱ��û�������ɣ�������Һ�к���H������a��bmol ���������ӣ���b��8mol �����۵������䣬��������������ӣ�����һ������NH4+����8��cmol �����������٣�������Һ��һ������Al3�������Dz�û���ܽ��꣬����ԭ��Һ�л���Mg2������˺��е�����Ϊ��H����Al3����NH4+ ��Mg2�����ܽ�2mol����������������������2mol������������Ϊ2mol����ôþ����Ϊ3mol��8mol�Ĺ�������������16mol���������ƣ� b��a��12mol��16��2��3��3��2��4mol��Ҳ����˵�������Ӹ�笠������ܵ����ʵ���Ϊ4mol��8mol�Ĺ������ƻ�����4mol����������˰���Ϊ2mol����ô笠�����Ϊ��2mol������������Ϊ2mol�����ԣ�n(H��)�sn(Al3��)�sn(NH4+)�s n(Mg2��)= 2:2:2:3����Һ��һ�������ڵ������ǣ�OH����CO32-��AlO2-����2��Al(OH)3+OH��= AlO2- +2H2O��2�֣�
���㣺��������֮��ķ�Ӧ��

��12�֣�ij�����Һ�п��ܺ��е��������±���ʾ��
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH ��Fe3+ ��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO ��AlO ��AlO |
��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)���������������Һ�������V���Ĺ�ϵ����ͼ��ʾ��
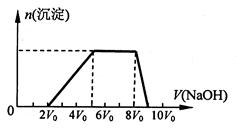
�ٸ���Һ��һ�������ڵ���������______________ ��һ�������ڵ���������__________��
���е����������Ӧ���ʵ���Ũ��֮��Ϊ__________________________________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
| Cl2���������״���� | 11.2 L | 22.4 L | 28.0 L |
| n (Cl-) | 2.5 mol | 3.5 mol | 4.0 mol |
| n (Br-) | 3.0 mol | 2.5mol | 2.0 mol |
| n (I-) | x mol | 0 | 0 |
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��
��12�֣�ij�����Һ�п��ܺ��е��������±���ʾ��
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH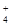 ��Fe3+ ��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO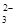 ��AlO ��AlO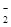 |
��1��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)���������������Һ�������V���Ĺ�ϵ��ͼ��ʾ��
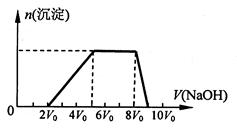
�ٸ���Һ��һ�����е���������______________�����Ӧ���ʵ���Ũ��֮��Ϊ________��һ�������ڵ���������_____________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��2��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
| Cl2���������״���� | 5.6 L | 11.2 L | 22.4 L |
| n (Cl-) | 2.5 mol | 3.0 mol | 4.0 mol |
| n (Br-) | 3.0 mol | 2.8 mol | 1.8 mol |
| n (I-) | x mol | 0 | 0 |
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��
ij�����Һ�п��ܺ��е��������±���ʾ��
|
���ܴ������е������� |
H+��Ag+��Mg2+��Al3+��NH |
|
���ܴ������е������� |
Cl-��Br-��I-��CO |
Ϊ̽����ɷ֣�����������̽��ʵ�顣
��1��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)��������� ������Һ�������V���Ĺ�ϵ��ͼ��ʾ��

�ٸ���Һ��һ�����е���������______________�����Ӧ���ʵ���Ũ��֮��Ϊ________��һ�������ڵ���������_____________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��2��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ�� ������ش��������⣺
|
Cl2���������״���� |
5.6 L |
11.2 L |
22.4 L |
|
n (Cl-) |
2.5 mol |
3.0 mol |
4.0 mol |
|
n (Br-) |
3.0 mol |
2.8 mol |
1.8 mol |
|
n (I-) |
x mol |
0 |
0 |
�ٵ�ͨ��Cl2�����Ϊ5.6 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ_______________��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��
��12�֣�ij�����Һ�п��ܺ��е��������±���ʾ��
|
���ܴ������е������� |
H+��Ag+��Mg2+��Al3+��NH |
|
���ܴ������е������� |
Cl-��Br-��I-��CO |
Ϊ̽����ɷ֣�����������̽��ʵ�顣
��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)���������������Һ�������V���Ĺ�ϵ����ͼ��ʾ��

�ٸ���Һ��һ�������ڵ���������______________ ��һ�������ڵ���������__________��
���е����������Ӧ���ʵ���Ũ��֮��Ϊ__________________________________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
|
Cl2���������״���� |
11.2 L |
22.4 L |
28.0 L |
|
n (Cl-) |
2.5 mol |
3.5 mol |
4.0 mol |
|
n (Br-) |
3.0 mol |
2.5mol |
2.0 mol |
|
n (I-) |
x mol |
0 |
0 |
�ٵ���ʼ��ͨ��Cl2�����Ϊ22.4Lʱ����Һ�з�����Ӧ�ܵ����ӷ���ʽΪ __________________________________________ ��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��
 ��Fe3+
��Fe3+ ��AlO
��AlO
 ��Fe3+
��Fe3+ ��AlO
��AlO