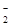��Ŀ����
ij�����Һ�п��ܺ��е��������±���ʾ��
|
���ܴ������е������� |
H+��Ag+��Mg2+��Al3+��NH |
|
���ܴ������е������� |
Cl-��Br-��I-��CO |
Ϊ̽����ɷ֣�����������̽��ʵ�顣
��1��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)��������� ������Һ�������V���Ĺ�ϵ��ͼ��ʾ��

�ٸ���Һ��һ�����е���������______________�����Ӧ���ʵ���Ũ��֮��Ϊ________��һ�������ڵ���������_____________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��2��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ�� ������ش��������⣺
|
Cl2���������״���� |
5.6 L |
11.2 L |
22.4 L |
|
n (Cl-) |
2.5 mol |
3.0 mol |
4.0 mol |
|
n (Br-) |
3.0 mol |
2.8 mol |
1.8 mol |
|
n (I-) |
x mol |
0 |
0 |
�ٵ�ͨ��Cl2�����Ϊ5.6 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ_______________��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��
(1)��H+��NH4+��Al3+��2��3:1��CO32‾��AlO2-
��Al(OH)3+OH‾= AlO2-+2H2O
(2)Cl2+2I‾=I2+2Cl‾;10:15:4
��������
�����������1���ٸ���ͼ��NaOH��Һ��������0-2V0ʱ����H+��Ӧ��2V0-4V0ʱ,NaOH��Al3+��Ӧ������������������6V0-8V0ʱ,����������NH4+��Ӧ���������ӷ���ʽ��������ʵ���Ũ��֮��Ϊ2:3:1����ΪAl3+��CO32‾��AlO2-��Ӧ�����ܹ��棬�������������Ӳ����ڡ��ڳ�������ʱ�����ķ�ӦΪNaOH��Al(OH)3�ķ�Ӧ��
��2���ٸ��ݻ�ԭ��ǿ����Cl2������I‾��Ȼ��������Br‾��ͨ��Cl2�����Ϊ5.6 Lʱ��I‾����ʣ�࣬Cl2ֻ����I‾����ԭ��Һ�е�Cl‾Ϊ��2.5mol-5.6L��22.4L/mol��2=2mol��n (Br‾)=3.0mol������Cl211.2Lʱ�����������е�I‾��0.2mol��Br‾��n(I‾)=2��(11.2L��22.4L/mol-1/2��0.2mol)=0.8mol,��ˣ�Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ10��15��4
���㣺���⿼�����ӵ��ƶϡ��������ʵ����ļ��㡢���ӷ���ʽ����д��������ԭ��Ӧ���Ⱥ�˳����ؼ��㡣

��12�֣�ij�����Һ�п��ܺ��е��������±���ʾ��
|
���ܴ������е������� |
H+��Ag+��Mg2+��Al3+��NH |
|
���ܴ������е������� |
Cl-��Br-��I-��CO |
Ϊ̽����ɷ֣�����������̽��ʵ�顣
��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)���������������Һ�������V���Ĺ�ϵ����ͼ��ʾ��

�ٸ���Һ��һ�������ڵ���������______________ ��һ�������ڵ���������__________��
���е����������Ӧ���ʵ���Ũ��֮��Ϊ__________________________________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
|
Cl2���������״���� |
11.2 L |
22.4 L |
28.0 L |
|
n (Cl-) |
2.5 mol |
3.5 mol |
4.0 mol |
|
n (Br-) |
3.0 mol |
2.5mol |
2.0 mol |
|
n (I-) |
x mol |
0 |
0 |
�ٵ���ʼ��ͨ��Cl2�����Ϊ22.4Lʱ����Һ�з�����Ӧ�ܵ����ӷ���ʽΪ __________________________________________ ��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��
 ��Fe3+
��Fe3+ ��AlO
��AlO
 ��Fe3+
��Fe3+ ��AlO
��AlO