��Ŀ����
����Ŀ��FeBr2�������л��ϳɵĴ�����ijУͬѧ���ʵ���ø����HBr��Fe��Ӧ�Ʊ�����FeBr2��ʵ��װ������(���ּг�װ����ʡ��)��
��֪������ʱFeBr3��ֽ�ΪFeBr2��FeBr2����ˮ���⣬800�����Ͽ�������
�ش��������⣺
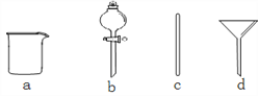
��1������M��������____________��װ�â�������HBr�Ļ�ѧ����ʽΪ___________��
��2����Ӧ��ʼǰͨ��N2��Ŀ����____________����Ӧ������ͨ��N2��Ŀ����____________��
��3������װ�âܵ������л�������Br2�Բ�Ʒ����_______(��С���û�С�)Ӱ�죬������___________��
��4��װ�â�������___________���ݳ���������Ҫ��___________(�ѧʽ)��
��5�����ʵ�鷽��̽���õ���FeBr2��Fe2+��Br���Ļ�ԭ��ǿ����___________________��
���𰸡�����Һ©�� NaBr��H3PO4![]() NaH2PO4��HBr�� ������������װ���еĿ����ž��� ϡ��HBr�������������װ�� û�� ���ɵ�����FeBr3 �ڸ����»�ֽ�ΪFeBr2 ����ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â� H2��N2 ȡ������Ʒ���ھ����������ȴ�������ˮ��ȡ��Һ����������KSCN������CCl4��������ˮ���������۲��������������𰸣�
NaH2PO4��HBr�� ������������װ���еĿ����ž��� ϡ��HBr�������������װ�� û�� ���ɵ�����FeBr3 �ڸ����»�ֽ�ΪFeBr2 ����ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â� H2��N2 ȡ������Ʒ���ھ����������ȴ�������ˮ��ȡ��Һ����������KSCN������CCl4��������ˮ���������۲��������������𰸣�
��������
��1������M�������Ƿ�Һ©���������ѻӷ�����H3PO4�ƻӷ�����HBr��������������ƣ�
��2��Ϊ��ֹ����������Ӧ����Ӧ��ʼǰͨ��N2��Ŀ���ǽ�װ���еĿ��������ž�����Ӧ������ͨ��N2��Ŀ����ϡ��HBr�������������װ�á�
��3������Ϣ��֪������ʱFeBr3��ֽ�ΪFeBr2���ʽ���װ�âܵ������л�������Br2�Բ�Ʒ����û��Ӱ�죻
��4���Ƶõ�HBr��Fe��Ӧ����FeBr2��������װ�â���װ�м�ʯ�ң�����ˮ���������壬�����dz�ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â����ݳ���������Ҫ�������͵�����
��5����ԭ��ǿ����Fe2+>Br������������ˮ����˭�ȷ�Ӧ��
��1������M�������Ƿ�Һ©���������ѻӷ�����H3PO4�ƻӷ�����HBr��������������ƣ�װ�â�������HBr�Ļ�ѧ����ʽΪNaBr��H3PO4![]() NaH2PO4��HBr����
NaH2PO4��HBr����
��2����Ӧ��ʼǰͨ��N2��Ŀ���ǽ�װ���еĿ��������ž�����ֹ����������Ӧ����Ӧ������ͨ��N2��Ŀ����ϡ��HBr�������������װ�á�
��3������Ϣ��֪������ʱFeBr3��ֽ�ΪFeBr2���ʽ���װ�âܵ������л�������Br2�Բ�Ʒ����û��Ӱ�죻
��4���Ƶõ�HBr��Fe��Ӧ����FeBr2��������װ�â���װ�м�ʯ�ң�����ˮ���������壬�����dz�ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â����ݳ���������Ҫ�������͵�������ѧʽΪH2��N2��
��5��Ҫ֤��Fe2+��Br���Ļ�ԭ�Ե�ǿ����ϵ�����Լ�������ˮ����˭�ȷ�Ӧ��ȡ������Ʒ���ھ����������ȴ�������ˮ��ȡ��Һ����������KSCN������CCl4��������ˮ�����������ú�۲��������������𰸣������ϲ���ҺΪѪ��ɫ����Fe2+>Br�������ϲ���Һ��Ϊdz��ɫ���²�Һ��Ϊ�Ⱥ�ɫ����Fe2+��Br����

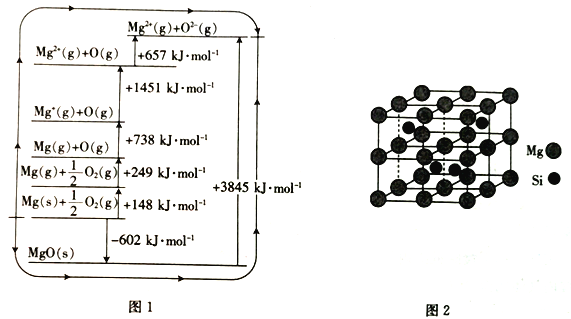
����Ŀ�����±��ṩ���ݼ�������ʽṹ֪ʶ����Ӧ1��SiCl4(g)+2H2(g)=Si(g)+4HCl(g)����Ӧ2��Si(g)+O2(g)=SiO2(g)����Ӧ1�ͷ�Ӧ2�ķ�Ӧ��Ϊ
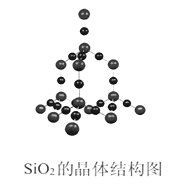 ��ľ���ṹ
��ľ���ṹ
��ѧ�� | Si-Cl | H-H | Si-Si | H-Cl | O=O | Si-O |
����kJ/mol�� | 360 | 436 | 176 | 431 | 498 | 460 |
A. +236kJ/mol��-990kJ/mol B. -116kJ/mol��-990kJ/mol
C. -116kJ/mol��-70kJ/mol D. +236kJ/mol��-70kJ/mol