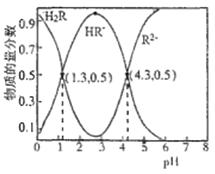
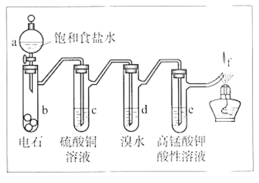
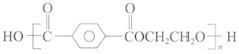
ћвƒњƒЏ»Ё
°Њћвƒњ°њMnO2”÷√ыЇЏ√ћњу£ђ÷ч“™”√”Џ…ъ≤ъ”≈÷ »ні≈ћъ—хће°£MnO2µƒЇѕ≥…ЈљЈ®∞і÷∆±Єє§“’÷–Ћщ”√‘≠Ѕѕµƒ≤їЌђ£ђЈ÷ќ™єћѕаЇѕ≥…ЇЌ“ЇѕаЇѕ≥…°£“—÷™£ЇMnO2≤ї»№”ЏЋЃ£ђ∆д÷–√ћµƒЉџћђ”–+2Љџ£ђ“≤њ…ƒ№”–+3ЉџЇЌ+4Љџ°£«лїЎірѕ¬Ѕ–ќ ћв£Ї
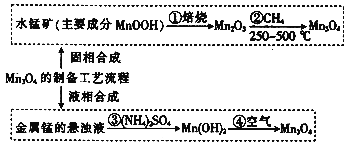
(1)»фMn3O4÷–√ћµƒЉџћђњі„ч”…+2ЇЌ+4Љџ„й≥…£ђ–і≥цЋь”…—хїѓќп–ќ≥…µƒ±ніп љ£Ї_____
(2)MnOOH÷–√ћµƒЉџћђќ™________Љџ£ђ–і≥цҐЏµƒїѓ—ІЈљ≥ћ љ£Ї____£їЉо–‘–њ√ћЄ…µз≥Ўµƒµз≥ЎЈі”¶ќ™£ЇZn+2MnO2 + 2H2O=Zn(OH)2 + 2MnOOH£ђ–і≥цЄ√µз≥Ў’эЉЂµƒµзЉЂЈі”¶ љ______
(3)љЂ(NH4)2SO4»№”ЏЋЃ є√ћµƒ–ь„«“Їѕ‘Ћб–‘£ђЋжЉіїЇ¬эµЎ≤ъ…ъ∆ш≈Ё£ђ ‘”√ѕа”¶µƒјл„”Јљ≥ћ љљв Ќ‘≠“т____________°£єэ¬Ћ≥цµƒMn(OH)2–и“™ѕіµ”£ђЉт“™Ћµ√чѕіµ”≥Ѕµнµƒ≤ў„чєэ≥ћ£Ї________°£
(4)»фҐџ÷– ’Љѓµљ672mL(±к„Љ„іњцѕ¬)µƒH2£ђ‘тјн¬џ…ѕњ…“‘µ√µљ_________g Mn3O4°£
°Њір∞Є°њ2MnO°§MnO2їтMnO2°§2MnO +3 12Mn2O3+CH4![]() 8Mn3O4+CO2+2H2O MnO2+e-+H2O=MnOOH+OH- Mn+2NH4++2H2O=Mn2++H2°ь+2NH3°§H2O ѕтєэ¬Ћ∆ч÷–Љ”’фЅуЋЃљю√ї≥Ѕµн£ђіэЋЃ„‘»їЅч≥цЇу£ђ÷ЎЄі…ѕ ц≤ў„ч2-3іќ 2.29
8Mn3O4+CO2+2H2O MnO2+e-+H2O=MnOOH+OH- Mn+2NH4++2H2O=Mn2++H2°ь+2NH3°§H2O ѕтєэ¬Ћ∆ч÷–Љ”’фЅуЋЃљю√ї≥Ѕµн£ђіэЋЃ„‘»їЅч≥цЇу£ђ÷ЎЄі…ѕ ц≤ў„ч2-3іќ 2.29
°Њљвќц°њ
”…÷∆±ЄЅч≥ћњ…÷™£ђMnOOH±Ї…’…ъ≥…Mn2O3£ђҐЏ÷–ЈҐ…ъ12Mn2O3+CH4![]() 8Mn3O4+CO2+2H2O£ђMn3O4÷–√ћµƒЉџћђњі„ч”…+2ЇЌ+4Љџ„й≥…£ђњ…–і≥…2MnOMnO2£ђљр ф√ћµƒ–ь„«“Ї”лЅтЋбпІЈҐ…ъЈі”¶ќ™Mn+2NH4++2H2O=Mn2++H2°ь+2NH3H2O£ђєэ¬Ћ≥цµƒMn(OH)2–и“™ѕіµ”£ђ‘Џњ’∆ш÷–Љ”»»…ъ≥…Mn3O4£ђ“‘іЋјіљвір°£
8Mn3O4+CO2+2H2O£ђMn3O4÷–√ћµƒЉџћђњі„ч”…+2ЇЌ+4Љџ„й≥…£ђњ…–і≥…2MnOMnO2£ђљр ф√ћµƒ–ь„«“Ї”лЅтЋбпІЈҐ…ъЈі”¶ќ™Mn+2NH4++2H2O=Mn2++H2°ь+2NH3H2O£ђєэ¬Ћ≥цµƒMn(OH)2–и“™ѕіµ”£ђ‘Џњ’∆ш÷–Љ”»»…ъ≥…Mn3O4£ђ“‘іЋјіљвір°£
(1)»фMn3O4÷–√ћµƒЉџћђњі„ч”…+2ЇЌ+4Љџ„й≥…£ђЋь”…—хїѓќп–ќ≥…µƒ±ніп љ2MnOMnO2їтMnO22MnO£ї
(2)MnOOH÷–Oќ™-2Љџ£ђHќ™+1Љџ£ђ”…їѓЇѕќп÷–’эЄЇїѓЇѕЉџµƒіъ эЇЌќ™0њ…÷™£ђ‘т√ћµƒЉџћђќ™+3£їҐЏµƒїѓ—ІЈљ≥ћ љќ™12Mn2O3+CH4![]() 8Mn3O4+CO2+2H2O£їЉо–‘–њ√ћЄ…µз≥Ўµƒµз≥ЎЈі”¶ќ™£ЇZn+2MnO2 + 2H2O=Zn(OH)2 + 2MnOOH£ђЄЇЉЂќ™£ЇZn-2e-+2OH-= Zn(OH)2£ђ’эЉЂЈі”¶ љњ…”…„№Јі”¶Љх»•ЄЇЉЂЈі”¶£ђЄ√µз≥Ў’эЉЂµƒµзЉЂЈі”¶ љMnO2+e-+H2O =MnOOH + OH-£ї
8Mn3O4+CO2+2H2O£їЉо–‘–њ√ћЄ…µз≥Ўµƒµз≥ЎЈі”¶ќ™£ЇZn+2MnO2 + 2H2O=Zn(OH)2 + 2MnOOH£ђЄЇЉЂќ™£ЇZn-2e-+2OH-= Zn(OH)2£ђ’эЉЂЈі”¶ љњ…”…„№Јі”¶Љх»•ЄЇЉЂЈі”¶£ђЄ√µз≥Ў’эЉЂµƒµзЉЂЈі”¶ љMnO2+e-+H2O =MnOOH + OH-£ї
(3)љЂ(NH4)2SO4»№”ЏЋЃ є√ћµƒ–ь„«“Їѕ‘Ћб–‘£ђЋжЉіїЇ¬эµЎ≤ъ…ъ∆ш≈Ё£ђ”√ѕа”¶µƒјл„”Јљ≥ћ љљв Ќ‘≠“тќ™Mn+2NH4++2H2O=Mn2++H2°ь+2NH3H2O£їєэ¬Ћ≥цµƒMn(OH)2–и“™ѕіµ”£ђЉт“™Ћµ√чѕіµ”≥Ѕµнµƒ≤ў„чєэ≥ћќ™ѕтєэ¬Ћ∆ч÷–Љ”’фЅуЋЃљю√ї≥Ѕµн£ђіэЋЃ„‘»їЅч≥цЇу£ђ÷ЎЄі…ѕ ц≤ў„ч2-3іќ£ї
(4)”…Mn+2NH4++2H2O=Mn2++H2°ь+2NH3H2O°Ґ6Mn(OH)2+O2![]() 2Mn3O4+6H2Oњ…÷™£ђіж‘ЏєЎѕµ3H2°ЂMn3O4£ђ‘тҐџ÷– ’Љѓµљ672mL(±к„Љ„іњцѕ¬)µƒH2£ђ‘тјн¬џ…ѕњ…“‘µ√µљMn3O4µƒ÷ Ѕњќ™
2Mn3O4+6H2Oњ…÷™£ђіж‘ЏєЎѕµ3H2°ЂMn3O4£ђ‘тҐџ÷– ’Љѓµљ672mL(±к„Љ„іњцѕ¬)µƒH2£ђ‘тјн¬џ…ѕњ…“‘µ√µљMn3O4µƒ÷ Ѕњќ™![]() °Ѕ
°Ѕ![]() °Ѕ229g/mol=2.29g°£
°Ѕ229g/mol=2.29g°£

 ‘ƒґЅњм≥µѕµЅ–ір∞Є
‘ƒґЅњм≥µѕµЅ–ір∞Є