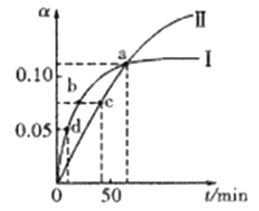
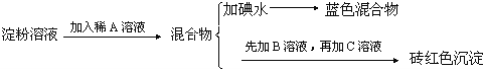
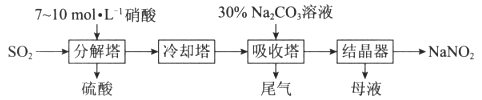
��Ŀ����
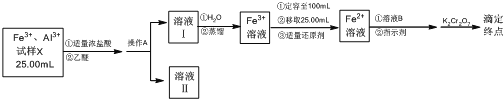
����Ŀ��ijͬѧ�������Ͻ���Ʒ�ܽ��ȡ��25.00 mL�����벢�ⶨFe3����Ũ�ȣ�ʵ�鷽��������£�
��֪������[(C2H5)2O]��һ���ӷ�����ȼ���������Ե��л�������е�Ϊ34.5 �棬����ˮ���ڽϸߵ�����Ũ���£�Fe3����HCl�������γɻ�����[(C2H5)2OH][FeCl4]���������ѣ�������Ũ�Ƚ���ʱ���û�������롣
��ش�
��1������A��������____________��
��2��������A��Ϊ�ж�Fe3���Ƿ��в����������ʵ�鷽��___________��
��3���ζ�ǰ�������������ҺB��________��
A��H2SO3H3PO4 B��H2SO4H3PO4 C��HNO3H3PO4 D��HIH3PO4
��4���ζ��ﵽ�յ�ʱ������0.100 0 mol��L��1 K2Cr2O7��Һ6.00 mL�����ݸ�ʵ�����ݣ�����X��c(Fe3��)Ϊ________��
��5�������ⶨ�������һ������Ϊ��߸õζ�����ľ��ܶȺ�ȷ�ȣ��ɲ�ȡ�Ĵ�ʩ��________��
A��ϡ�ͱ������� B�����ٱ�������ȡ��
C������ƽ�вⶨ���� D�����͵ζ���Ũ��
���𰸡� ��Һ ����Һ����ȡ�����μ�KSCN��Һ����Ѫ��ɫ˵������Fe3��������ɫ˵������ B 0.576 mol��L��1 CD
����������Fe3+��Al3+����X��Һ25mL����������Ũ��������ѣ��ڽϸߵ�����Ũ���£�Fe3+��HCl�������γɻ�����[(C2H5)2OH][FeCl4]���������ѣ���������ˮ���ֲ��ͨ����Һ����õ���Һ��ΪAl3+����Һ������Һ���������γɻ�����[(C2H5)2OH][FeCl4]����Һ��������Ũ�Ƚ���ʱ���û�������룬����ˮ�����ͨ������ȥ���ѣ��õ��������ӵ�ˮ��Һ��������100mL��ȡ25.00mL��Һ������������ԭ���õ�Fe2+���ӵ���Һ������ָʾ�����ζ�ǰ�������������ҺBΪ�����������Ӻͻ�ԭ�Ե��ᣬ���ظ������Һ�ζ����յ㣬�ݴ˽��
��1���������Ϸ�����֪����A�������ڽϸߵ�����Ũ���£�Fe3+��HCl�������γɻ�����[(C2H5)2OH][FeCl4]���������ѣ���������ˮ���ֲ��ͨ����Һ����õ���Һ��ΪAl3+����Һ������Һ���������γɻ�����[(C2H5)2OH][FeCl4]����Һ������A������Ϊ��Һ��
��2����������������KSCN��Һ���ɫ���ʵ����������ӵĴ��ڣ�������Һ����ȡ�����μ�KSCN��Һ����Ѫ��ɫ˵������Fe3��������ɫ˵��������
��3���ζ���Ҫ�����Ի�����������ܾ��л�ԭ�ԡ������ԣ����ܱ�������������Ҳ���ܱ��������ӻ�ԭ����
A��H2SO3-H3PO4����������л�ԭ�ԣ�Ҳ�ᱻ���������������ı���Һ��������A����
B��H2SO4-H3PO4����Ϊ���������ᣬ�����ṩ���Ի����Ҳ�Ӱ��ⶨ��Ӧ��B��ȷ��
C��HNO3-H3PO4���������ǿ�����ԣ����������������ӣ����µ�������������Һ���٣�������C����
D��HI-H3PO4��HI���ǻ�ԭ���ᣬҲ�������ĵ���������������ı���Һ��������D����
��ΪB��
��4���������ӷ�Ӧ������ϵ���㣬���ﵽ�յ�ʱ������0.1000 molL-1K2Cr2O7��Һ6.00mL�����ʵ���=0.1000mol/L��0.00600L=0.00060mol����ӦΪ
Cr2O72-+6Fe2++H+=2Cr3++6Fe3++7H2O
1 6
0.00060mol 0.00360mol
c=0.00360mol��0.0250L=0.144mol/L
������X��c��Fe3+��=0.144mol/L��100/25=0.576mol/L��
��5�������ⶨ�������һ������Ϊ��߸õζ�����ľ��ܶȺ�ȷ�ȣ������ظ�����ʵ�飬��ֵȡƽ��ֵ���������ζ���ҺŨ��ԽС���ⶨ���Խȷ����
A��ϡ�ͱ���������Ũ�ȼ�С���ⶨ��������A����
B�����ٱ�������ȡ�����͵ζ�����ľ��ܶȺ�ȷ���أ�B����
C������ƽ�вⶨ���������ٲⶨ���������������ȷ�ȣ�C��ȷ��
D�����͵ζ���Ũ�ȣ���Ӧ�յ��жϸ�ȷ���ζ�����ľ��ܶȸߣ�D��ȷ��
��ΪCD��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д�