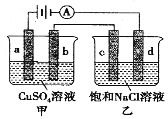
��Ŀ����
����Ŀ����ҵ��ˮ�г�����һ����Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ��˺���
��֪��i. 2CrO42-����ɫ��+ 2H+ ![]() Cr2O72-����ɫ��+ H2O
Cr2O72-����ɫ��+ H2O
ii. Cr(OH)3(s) + H2O ![]() [Cr(OH)4]- (����ɫ) + H+
[Cr(OH)4]- (����ɫ) + H+
(1) ������ҵ��ˮ��һ�ִ����������£�
![]()
�� i. ����ת��һ���е�����Һ��pH=2������Һ��_______ɫ��
ii. ��˵��ת��һ����Ӧ��ƽ��״̬����_______������ĸ��ţ���
a��Cr2O72-��CrO42-��Ũ����ͬ b������(Cr2O72-) = 2����(CrO42-)
c����Һ����ɫ���� d����Һ��pH����
�� ����ڻ�ԭһ�������ӷ���ʽ��___________________________������ԭl mol Cr2O72-���ӣ���ҪFeSO4��7H2O�����ʵ�����_______mol��
�� ����һ���У���Cr3+(��ɫ)��Һ�У��μ�NaOH��Һ����pH��4.6ʱ����ʼ���ֻ���ɫ����������pH�����ߣ����������ࡣ��pH��13ʱ����������ʧ����Һ��Ϊ����ɫ��
i. �������Һ��Ϊ����ɫ��ԭ��_______��
ii. ����0.05mol��L-1��Cr2(SO4)3��Һ50mL�У�һ���Լ�������0.6 mol��L-1��NaOH��Һ����ַ�Ӧ����Һ�пɹ۲쵽��������__________��
�� ��K[Cr(OH)4]��K2Cr2O7�����Һ�м�������H2SO4�ữ����Ԫ����_______��ʽ���ڣ������ӷ��ţ���
(2) ��Fe���缫��⺬Cr2O72-�����Թ�ҵ��ˮ������ֱ�ӳ�ȥ�������ŵ����У�������������ҺpH���ߣ�����Cr(OH)3������
�� ���������ҵ���̷�����Fe���缫��ԭ��_______��
�� ��ϵ缫��Ӧʽ����������������ҺpH���ߵ�ԭ��_______��
�� ��Һ��ͬʱ���ɵij������ܻ���_______(�ѧʽ)��
���𰸡� �� cd ![]() 6 ������ҺpH����ƽ�� Cr(OH)3(s) + H2O
6 ������ҺpH����ƽ�� Cr(OH)3(s) + H2O ![]() [Cr(OH)4]- (����ɫ) + H+�����ƶ�����Һ��Ϊ����ɫ ��Һ����ɫ���ձ�Ϊ����ɫ Cr3+��Cr2O72- ����������Ӧ������ӦFe - 2e- == Fe2����Ϊ��ԭCr2O72-�ṩ��ԭ��Fe2�� ����������Ӧ2H��+2e- == H2�� ����c(H��)�½� Fe(OH)3
[Cr(OH)4]- (����ɫ) + H+�����ƶ�����Һ��Ϊ����ɫ ��Һ����ɫ���ձ�Ϊ����ɫ Cr3+��Cr2O72- ����������Ӧ������ӦFe - 2e- == Fe2����Ϊ��ԭCr2O72-�ṩ��ԭ��Fe2�� ����������Ӧ2H��+2e- == H2�� ����c(H��)�½� Fe(OH)3
��������(1)��i.c(H+)����ƽ��2CrO42-(��ɫ)+2H+Cr2O72-(��ɫ)+H2O���ƣ���Һ�ʳ�ɫ��
ii. �����ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣻a��Cr2O72-��CrO42-��Ũ����ͬȡ������ʼŨ�Ⱥ�ת���������ж�ƽ�⣬��A����b��2v(Cr2O72-)=v(CrO42-)�������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��B����c����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣬��C��ȷ���ʴ�Ϊ��C��d����Һ��pH���䣬Ϊ�����������ж�ƽ�⣬��d��ȷ����Ϊcd��
����������Fe2+�����������»�ԭCr2O72-�õ�Cr3+�����ӷ���ʽΪ6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O�����ݵ��ӵ�ʧ�غ㣺n(Cr2O72-)��6=n(FeSO47H2O)��1��n(FeSO47H2O)= ![]() =6mol��
=6mol��
��i.��Cr3+(��ɫ)��Һ�У��μ�NaOH��Һ��������Ũ�ȼ�С����ҺpH����ƽ��Cr(OH)3(s)+H2O![]() [Cr(OH)4]-(����ɫ)+H+�����ƶ�������Һ��Ϊ����ɫ��
[Cr(OH)4]-(����ɫ)+H+�����ƶ�������Һ��Ϊ����ɫ��
ii.����0.05molL-1��Cr2(SO4)3��Һ50mL�У�һ���Լ�������0.6molL-1��NaOH��Һ����������Ũ�Ƚϴ��ҹ�����ƽ�������Ƴ��ף���Һ����ɫ���ձ�Ϊ����ɫ��
����K[Cr(OH)4]��K2Cr2O7�����Һ�м�������H2SO4�ữ��i.2CrO42-(��ɫ)+2H+![]() Cr2O72-(��ɫ)+H2O��ii��Cr(OH)3(s)+H2O
Cr2O72-(��ɫ)+H2O��ii��Cr(OH)3(s)+H2O![]() [Cr(OH)4]-(����ɫ)+H+��������Ũ���㹻��i�����ƣ�ii�����ƣ����������ܽ⣬�õ�Cr3+�����Ը�Ԫ����Cr3+��Cr2O72-���ڣ�
[Cr(OH)4]-(����ɫ)+H+��������Ũ���㹻��i�����ƣ�ii�����ƣ����������ܽ⣬�õ�Cr3+�����Ը�Ԫ����Cr3+��Cr2O72-���ڣ�
(2)������������Ӧ������ӦFe-2e-�TFe2+��Ϊ��ԭCr2O72-�ṩ��ԭ��Fe2+����Ӧ����Fe��������
����Һ���������������ŵ�����������������������������Ũ�Ƚ��ͣ���Һ��pH���ߣ��缫��ӦʽΪ2H++2e-=H2 ����
���������ӱ���������Fe3+��Fe3+��OH-��Ӧ����Fe(OH)3������