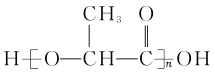��Ŀ����
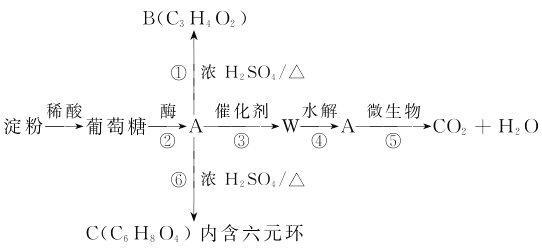
����Ŀ����֪�ס��ҡ���Ϊ�������ʣ�A��B��C��D��E��F��G��X��Ϊ�����Ļ����B��X��Ħ��������ͬ��E����Է���������D����Է���������16����һ�������£��������ת����ϵ����ͼ��ʾ��

(1)д��X��G�Ļ�ѧʽ��X________��G________��
(2)д���йر仯�Ļ�ѧ����ʽ��
D������______________________________��
C��G��_______________________________��
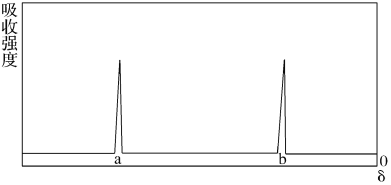
(3)д�����ӷ���ʽB��H2O��_________________��������0.5mol Bת�Ƶ��� mol ��
���𰸡���1��Na2S��2�֣�SO3
(2)2Na2SO3��O2��2Na2SO4��2�֣�
��SO3��2NaOH��Na2SO4��H2O��2�֣�
(3)2Na2O2��2H2O��4Na+��4OH- ��O2����2����0.5��2����
��������
������������ʼס��Ҿ��ֱܷ��뵥�ʱ��������η�Ӧ�������ǵ��ʼס��ҷֱ�O2�����������������ɲ�ͬ������������O2��������B��ˮ��Ӧ�ܷų�O2����B��Na2O2����ôC��NaOH��A��Na2O������Na������B��X��Ħ��������ͬ����X��Na2S����ô����S��F��SO2��G��SO3����һ���Ƴ�D��Na2SO3��E��Na2SO4����Na2SO4����Է���������Na2SO3����Է���������16���������⡣
��1��XΪNa2S��GΪSO3��
��2��D�ͱ��ķ�ӦΪ2Na2SO3��O2��2Na2SO4��C��G�ķ�ӦΪSO3��2NaOH��Na2SO4��H2O��
��3������������ˮ��Ӧ�����ӷ���ʽΪ2Na2O2��2H2O��4Na+��4OH- ��O2������������ʽ��֪������0.5mol �������ƣ�ת�Ƶ���0.5mol��