��Ŀ����
����Ŀ��ij�о���ѧϰС��ļס���ͬѧ�ֱ����������ʵ������֤Ԫ�������ɡ�
����ͬѧ���ƣ�þ������1mol�ֱ�Ͷ�뵽�����������У�Ԥ��ʵ������
��1����ͬѧ���ʵ���Ŀ����__________________________
��2����Ӧ����ҵ���______________
��3����NaOH��Һ��NH4Cl��Һ�������NH3.H2O,�Ӷ���֤NaOH�ļ���ǿ��NH3.H2O���̶���֤Na�Ľ����Դ���N������Ϊ������Ƿ������____________��˵�����ɣ�_____________.
������ͬѧ�������ͼװ����̽��̼����Ԫ�صķǽ�����ǿ��������Ҫ��������и�С��
��1��ʵ��װ�ã�
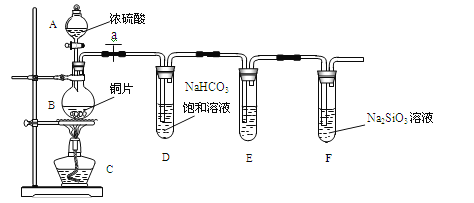
��д��ʾ��������A_________________B______________
��2��ʵ�鲽�裺 ����������____________����ҩƷ��a��Ȼ�����Ũ���ᣬ���ȡ�
��3������̽��������֪����ǿ��:������>̼�ᣩ
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��________________________;
��װ��E�е��Լ�Ϊ_________________________��������______________________����Ӧ����ʽ_____________________________________________;
����˵��̼Ԫ�صķǽ����Աȹ�Ԫ�طǽ�����ǿ��ʵ��������_________________________��
���𰸡� ��֤��þ��������(��ͬһ���ڣ�������Ԫ�صĽ���������) �� ������ �ü���ǿ���ȽϽ�����ǿ��ʱ��һ��Ҫ��Ԫ������������Ӧˮ����ļ���ǿ�����Ƚ� ��Һ©�� Բ����ƿ ���װ�õ������� ![]() ����KMnO4 ��ȥSO2���� 2KMnO4 +5SO2+2H2O=2MnSO4 +K2SO4 +2H2SO4 ʢ��Na2SiO3��Һ���Թ��г��ְ�ɫ����
����KMnO4 ��ȥSO2���� 2KMnO4 +5SO2+2H2O=2MnSO4 +K2SO4 +2H2SO4 ʢ��Na2SiO3��Һ���Թ��г��ְ�ɫ����
��������(��)(1)��ͬѧ���ƣ�þ������1mol�ֱ�Ͷ�뵽��������������Ŀ������֤��þ�������ԣ��ʴ�Ϊ����֤��þ�������ԣ�
(2)�ƵĻ�������ǿ����Ӧ����ҵ����ƣ��ʴ�Ϊ������
(3)��NaOH��Һ��NH4Cl��Һ�������NH3.H2O,�Ӷ���֤NaOH�ļ���ǿ��NH3.H2O���̶���֤Na�Ľ����Դ���N��������Ʋ���������ΪNH3.H2O���ǵ�Ԫ�ص�����������Ӧˮ����ʴ�Ϊ�����������ü���ǿ���ȽϽ�����ǿ��ʱ��һ��Ҫ��Ԫ������������Ӧˮ����ļ���ǿ�����Ƚϣ�
(��)(1)����װ��ͼ������AΪ��Һ©��������BΪԲ����ƿ���ʴ�Ϊ����Һ©����Բ����ƿ��
(2)������ͬѧ̽��̼����Ԫ�صķǽ�����ǿ����Ŀ�ģ�Ũ������ͭ��Ӧ���ɵĶ�������ͨ��̼��������Һ�з�Ӧ���ɶ�����̼����������̼�еĶ��������ȥ��ͨ���������Һ�м��ɣ����ʵ�鲽��Ϊ�� �������������װ�õ������ԡ���ҩƷ��a��Ȼ�����Ũ���ᣬ���ȣ��ʴ�Ϊ�����װ�õ������ԣ�
(3)��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��2H2SO4(Ũ) + Cu![]() CuSO4 + 2H2O +SO2�����ʴ�Ϊ��2H2SO4(Ũ) + Cu
CuSO4 + 2H2O +SO2�����ʴ�Ϊ��2H2SO4(Ũ) + Cu![]() CuSO4 + 2H2O +SO2����
CuSO4 + 2H2O +SO2����
��װ��E��Ŀ���dz�ȥ������̼�еĶ��������ݶ��ߵ����ʵIJ�𣬿���ѡ������KMnO4����������������ȥ����Ӧ����ʽΪ2KMnO4 +5SO2+2H2O=2MnSO4 +K2SO4 +2H2SO4���ʴ�Ϊ������KMnO4����ȥSO2������2KMnO4 +5SO2+2H2O=2MnSO4 +K2SO4 +2H2SO4��
����˵��̼Ԫ�صķǽ����Աȹ�Ԫ�طǽ�����ǿ��ʵ������Ϊʢ��Na2SiO3��Һ���Թ��г��ְ�ɫ�������ʴ�Ϊ��ʢ��Na2SiO3��Һ���Թ��г��ְ�ɫ������

����Ŀ��
��1�����ݼ�����������ƽ���ȡ�Ȼ���_________ g��
��2��������Һʱ������Ҫ�ձ����������⣬�������õ��IJ��������� ��
��3��������Һ�����м���������a���ܽ⣬b��ҡ�ȣ�c��ϴ�ӣ�d����ȴ��e��������f������Һ��������ƿ��g�����ݣ���ȷ�IJ���˳���� ��
��4�����в������ʹ��Һ���ʵ���Ũ��ƫ�͵���___ ______ ��
A��û�н�ϴ��Һת������ƿ�� |
B����ˮ����ʱ��ˮ���������˿̶��� |
C������ʱ����������ƿ�Ŀ̶��� |
D������ƿϴ�Ӻ�δ�����ﴦ�� |
��5��ȡ����NaCl��Һ10mL��ˮϡ�͵�200mL��ϡ�ͺ���Һ��NaCl�����ʵ���Ũ����_________