��Ŀ����
�⻯��ͭ(CuH)��һ���������ʣ���CuSO4��Һ�͡���һ���ʡ���40��50��ʱ��Ӧ����������CuH���е������У����ȶ����ֽ⣻����������ȼ�գ���ϡ���ᷴӦ���������壻Cu�������������·����ķ�Ӧ�ǣ�2Cu��=Cu2����Cu��
����������Ϣ������Լ������յĻ�ѧ֪ʶ���ش��������⣺
(1)��CuSO4��Һ�͡���һ���ʡ���CuH�ķ�Ӧ�У���������ԭ�۵�������⡰��һ���ʡ��ڷ�Ӧ����______(�����������ԭ����)��
(2)д��CuH��������ȼ�յĻ�ѧ��Ӧ����ʽ��________________________��
(3)CuH�ܽ���ϡ���������ɵ�������______(�ѧʽ)��
(4)�����CuH�ܽ���������ϡ���������ɵ�����ֻ��NO����д��CuH�ܽ�������ϡ�����з�Ӧ�����ӷ���ʽ��_____________________________________��
����������Ϣ������Լ������յĻ�ѧ֪ʶ���ش��������⣺
(1)��CuSO4��Һ�͡���һ���ʡ���CuH�ķ�Ӧ�У���������ԭ�۵�������⡰��һ���ʡ��ڷ�Ӧ����______(�����������ԭ����)��
(2)д��CuH��������ȼ�յĻ�ѧ��Ӧ����ʽ��________________________��
(3)CuH�ܽ���ϡ���������ɵ�������______(�ѧʽ)��
(4)�����CuH�ܽ���������ϡ���������ɵ�����ֻ��NO����д��CuH�ܽ�������ϡ�����з�Ӧ�����ӷ���ʽ��_____________________________________��
(1)��ԭ��
(2)2CuH��3Cl2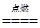 2CuCl2��2HCl
2CuCl2��2HCl
(3)H2
(4)CuH��3H���� =Cu2����2H2O��NO��
=Cu2����2H2O��NO��
(2)2CuH��3Cl2
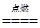 2CuCl2��2HCl
2CuCl2��2HCl(3)H2
(4)CuH��3H����
 =Cu2����2H2O��NO��
=Cu2����2H2O��NO����CuSO4������һ���ʡ���CuH֪����Ӧ��ͭԪ�ؼ�̬���ͣ��ʡ���һ���ʡ��ǻ�ԭ����CuH��ͭ����Ԫ�ؾ����ڵͼ�̬����ǿ������������Ӧʱ�����ɱ�����������CuCl2��HCl��CuH����ϡ����ʱ����Ԫ�ػᷢ����̬�����͵�������ԭ��Ӧ����������CuH��ϡ���ᷴӦ�ᱻ������Cu2����H��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ���������������л�ԭ��
���������������л�ԭ�� ______ �� ______ �� N2O�� �� H2O
______ �� ______ �� N2O�� �� H2O Cu(NO3)2
Cu(NO3)2 CuO
CuO Cu(OH)2
Cu(OH)2 CuSO4
CuSO4 Cu(NO3)2
Cu(NO3)2
