��Ŀ����
����ʵ������ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ����Fe��NO3��2��Ʒ�ѱ��������� | ��Fe��NO3��2��Ʒ����ϡH2SO4�μ�KSCN��Һ |
B | ��ȡ��ˮ�е� | ����ˮ�����Һ©�������������Ҵ������� |
C | ��֤Mg��OH��2��������ת��Ϊ Fe��OH��3���� | �� 2 mL1mol��L-1NaOH ��Һ�м���2 ~3 �� lmol��L-1MgCl2��Һ�����ɰ�ɫ�������ټ���2~3��lmol��L-1FeCl3��Һ |
D | �Ƚ�̼��ķǽ�����ǿ�� | �͵�Na2SiO3��Һ��ͨ��CO2 |
 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д���һ�����á���ʵ�ֶ��Ļ�ѧʵ��̽����ij����С��ѧ������ͼ15��ʾ��������ϣ�ʡ�Լгֺ;���װ�ã����������ʵ�飬�ش��������⣺
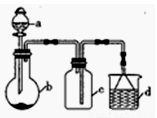
��1������a������Ϊ______________������a��ʹ��ǰҪ________________��
��2����װ�ÿ�����ijЩ�������ȡ���ռ���β�������b�ã��±���3��ʵ�����Ʒ�����������_________________������ţ�
ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
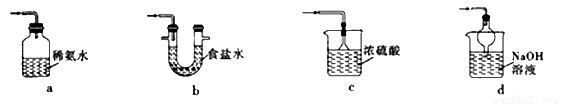
A | ϡ���� | Cu | NO | H2O |
B | ϡH2SO4 | CaCO3 | CO2 | NaOH��Һ |
C | Ũ��ˮ | NaOH���� | NH3 | H2O |
��3�������������Ӵ�ʱ��������̣���д����Ӧ�Ļ�ѧ����ʽ��_______________________��
��4������װ����a�е�����ΪŨ���ᣬb�е�����ΪCuƬ���ڼ���������Ҳ����ȡSO2��
������װ�ÿ�����SO2β���������ǣ��г���������ȥ��_____________������ţ���
��Fe2��SO4��3��ҺҲ�����ն����SO2����.д��SO2��Fe2��SO4��3��Һ��Ӧ�����ӷ���ʽ��_______________________��
��ijС���÷�Ӧ������CuSO4��Һ����ȡ�������ⶨ���õ�����CuSO4��xH2O���нᾧˮx��ֵ������ �������������±���
���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
������g�� | m1=5.4 | m2=8.2 | m3=7.4 | m4=7.2 | m5=7.2 |
��Ӧ����____________�����������ƣ��м��ȣ�����Ҫ���к��ز�����ԭ����__________________��CuSO4��xH2O�е�x=___________������1λС���������ⶨ���xƫ���ܵ�ԭ����____________������ţ���
a�������¶ȹ��� b����������Ŀ����ϴ� c�����Ⱥ���ڿ�������ȴ


 H++B2-���ش��������⣺
H++B2-���ش��������⣺ CH3OH��g��
CH3OH��g��

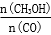 �������____________��
�������____________�� CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ���ǣ� ��
CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ���ǣ� ��
 ���пհף�
���пհף� CH3OH��g����
CH3OH��g����
 Ϊ0.1NA
Ϊ0.1NA