��Ŀ����
����Ŀ����ͼ�Ƕ����ѽ��ʵ�����̣�����ʾ��������һ���������ѽ�Ŀ��ܷ���ʽΪ��C4H10![]() C2H6+C2H4�� C4H10
C2H6+C2H4�� C4H10![]() CH4+C3H6��
CH4+C3H6��
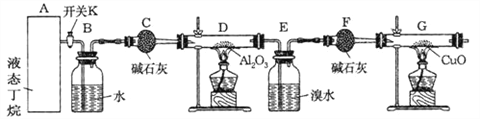
���Ӻ�װ�ú�����е�ʵ������У�
�ٸ�D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ�������
��1���������������Ⱥ�˳��������_______________________������ţ�
��2��д������������ͭ��Ӧ�Ļ�ѧ����ʽ______________________
��3������Eװ���еĻ�����ˮ���������ٰ���������ʵ�飺
�ٷ�����������������Ʒֱ��ǣ���________��________��Na2SO3��Һ�������ǣ������ӷ���ʽ��ʾ��________________________________________________________��
����֪B��̼ԭ��������A��̼ԭ��������д��B�Ľṹ��ʽ_____________________��
��4���ٶ�������ȫ�ѽ�������E+F��װ�õ��������ȷ�Ӧǰ������0��7 g�� Gװ�õ�����������1��76 g��������ѽ�����м������������ʵ���֮�� n��CH4��: n��C2H6��=__________��
���𰸡� ���� ���� �� CH4+4CuO![]() CO2+2H2O+4Cu ��Һ ����
CO2+2H2O+4Cu ��Һ ���� ![]()
![]() 1��1
1��1
����������K������ͨ��B��Bװ���Ǹ������ݿ����������٣�Cװ�ø��ﶡ�飬�������������������¶��鷢���ѽⷴӦ����ϩ����������E����ˮ����ϩ����F����������G��������Cu�ڼ��������·���������ԭ��Ӧ����Cu��
(1)Ӧ�ȼ��������ԣ��ϳ��ڲ����壬�ٸ�D��Gװ�ü��ȣ��ʴ�Ϊ���ڢۢ���
(2)�����������������������£����������ͭ��Ӧ���ɶ�����̼��ˮ��ͭ����Ӧ����ʽΪ��CH4+4CuO![]() CO2+2H2O+4Cu���ʴ�Ϊ��CH4+4CuO
CO2+2H2O+4Cu���ʴ�Ϊ��CH4+4CuO![]() CO2+2H2O+4Cu��
CO2+2H2O+4Cu��
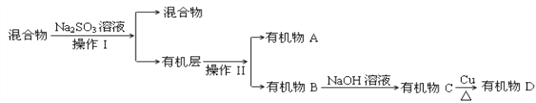
(3)������к����塢ˮ��������������������ƣ��������Ʊ����������������ƣ�ͬʱ����NaBr���Ӷ���ȥ�壬Ȼ����÷�Һ�������룬���л�����з���õ��л���A���л���B�����л����м���NaOH��Һ���õ��л���C��C�ܷ���������Ӧ����B����ˮ�ⷴӦ����CΪ����C���������õ�ȩD��
��ͨ�����Ϸ���֪�����������͢�����Ʒֱ��ǣ����Һ���������������ƾ��л�ԭ�ԣ��ܺ�ǿ�����������巴Ӧ����ȥ�壬���ӷ���ʽΪ��SO32-+Br2+H2O=SO42-+2Br-+2H+���ʴ�Ϊ����Һ������SO32-+Br2+H2O=SO42-+2Br-+2H+��
����֪B��̼ԭ��������A��̼ԭ������˵��B��̼ԭ�Ӹ�����3��A��̼ԭ�Ӹ�����2��BΪ1��2-������飬B�Ľṹ��ʽCH2BrCHBrCH3���ʴ�Ϊ��CH2BrCHBrCH3��
(4)������ѽ������ɵ���ϩ����������ʵ�����ȣ����ɵļ���ͱ�ϩ�����ʵ�����ȣ�E��F���յ���ϩ����G���ٵ�����������ͭ�е���Ԫ����������xΪC2H4�����ʵ�����yΪC3H6�����ʵ�����������ͼ�������ʵ����ֱ���x��y��28x+42y=0.7g������ͼ��������ͭ��Ӧ��Ҫ����ԭ�ӵ����ʵ���Ϊ2(2x+y)+![]() =
=![]() ����ã�x=y=0.07mol���ʴ�Ϊ��1��1��
����ã�x=y=0.07mol���ʴ�Ϊ��1��1��

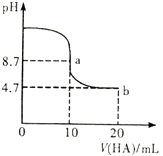
����Ŀ����֪������Ũ��Ϊ0.lmol/L��������Һ��pH���±��������й�˵����ȷ����
���� | NaF | NaClO | Na2CO3 |
pH | 7.5 | 9.7 | 11.6 |
A. ����ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳�� H2CO3<HClO<HF
B. �����ϱ���ˮ�ⷽ��ʽClO-+H2O![]() HClO+OH-��ˮ�ⳣ��K=10-7.6
HClO+OH-��ˮ�ⳣ��K=10-7.6
C. ����CO2ͨ��0.lmol/LNa2C03��Һ������Һ���ԣ�����Һ��2c(CO32-)+c(HCO3-)=0.1 mol/L
D. ������NaC1O��Һ��ͨHF������ǡ����ȫ��Ӧʱ��c(Na+)>c(F-)>(H+)>c(HClO)>c(OH-)