��Ŀ����
ij�¶��£����������������н��з�Ӧ��CO(g)+2H2(g) 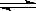 CH3OH(g) ��H1=��90.7 kJ•mol-1����֪��Ӧ����ʼŨ�ȷֱ�Ϊ��c(CO)=1 mol/L��c(H2)=2.4 mol/L��5 min��Ӧ��ƽ�⣬��ʱCO��ת����Ϊ50%��������˵����ȷ���ǣ�( )
CH3OH(g) ��H1=��90.7 kJ•mol-1����֪��Ӧ����ʼŨ�ȷֱ�Ϊ��c(CO)=1 mol/L��c(H2)=2.4 mol/L��5 min��Ӧ��ƽ�⣬��ʱCO��ת����Ϊ50%��������˵����ȷ���ǣ�( )
A���÷�Ӧ�����������ȷ�Ӧ��һ�����Է�����
B��5 min��H2��ƽ����Ӧ����Ϊ0.1 mol/(L��min)
C�������¶��£���Ӧ�����ʼŨ��c(CO)=4 mol/L��c(H2)=a mol/L���ﵽƽ���c(CH3OH)=2 mol/L����a=5.4
D�������������������£�������������CO��ת����
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�ij����С����ʹ������к͵ζ����ⶨij���۰״�������(g/100 mL)��
��.ʵ�鲽�裺��1����________(����������)��ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100 mL����ƿ�ж��ݣ�ҡ�ȼ��ô���״���Һ��

��2������ʽ�ζ���ȡ����״���Һ20.00 mL����ƿ�У������еμ�2��______��ָʾ����
��3����ȡʢװ0.1000 mol��L��1 NaOH ��Һ�ļ�ʽ �ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
��4���ζ��� ���������һ����Һ��Һ������ɫ��Ϊdz��ɫ����30�����ޱ仯ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��.ʵ���¼��
�ζ�����ʵ������(mL) | 1 | 2 | 3 | 4 |
V(��Ʒ) | 20.00 | 20.00 | 20.00 | 20.00 |
V(NaOH)(����) | 15.95 | 15.00 | 15.05 | 14.95 |
��.���ݴ��������ۣ�
��1����ʵ���������ݣ����۰״���������________g/100 mL��
��2���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����________(��д���)��
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ��������������Һ����


 ʽ֬����(trans fatty acids���TFA)�ĺ���������1/3���й�ʳ������������ʽ֬����Ԥ�����桱������ƣ��������ķ�ʽ֬���ᣬ������Ѫ�ܶ���������Ѫ�ܼ�����֬����Ľṹ����ͼ��ʾ��
ʽ֬����(trans fatty acids���TFA)�ĺ���������1/3���й�ʳ������������ʽ֬����Ԥ�����桱������ƣ��������ķ�ʽ֬���ᣬ������Ѫ�ܶ���������Ѫ�ܼ�����֬����Ľṹ����ͼ��ʾ��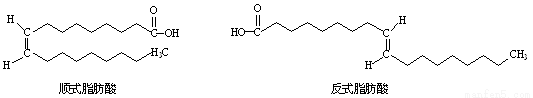

 ����Դ���� c���Ͼɸɵ��
����Դ���� c���Ͼɸɵ�� �� ��
�� �� ����Ʒd ������ԡ������ԡ����������á�
����Ʒd ������ԡ������ԡ����������á� B��ȩ���ĵ���ʽ��
B��ȩ���ĵ���ʽ��