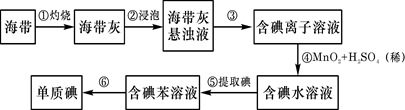
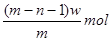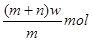��Ŀ����
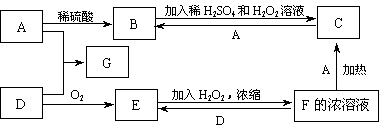
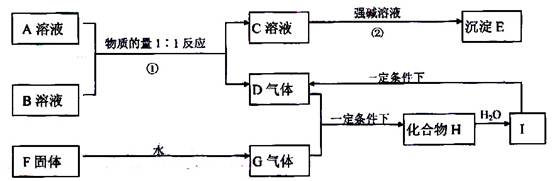
��12�֣�A��B��C��D��E��F�������ʵ�ת����ϵ��ͼ��ʾ(��Ӧ�����Ͳ��ֲ���δ���)
(1)��AΪ�����ڽ������ʣ�DΪ�����ڷǽ������ʣ�������Ԫ�ص�ԭ������A��D��2��������Ԫ�ص�ԭ������������D��A��2����F��Ũ��Һ��A��D��Ӧ���к���ɫ�������ɣ�
��A��ԭ�ӽṹʾ��ͼΪ__________����Ӧ�ܵĻ�ѧ����ʽΪ�� ��
(2)��AΪ�����Ľ������ʣ�D��F����̬���ʣ���Ӧ����ˮ��Һ�н��У���Ӧ��(��ˮ��Һ�н���)�����ӷ���ʽ��____________________����֪������1g D��F��Ӧ����B(��̬)ʱ�ų�92.3kJ������д����Ӧ���Ȼ�ѧ����ʽ____________________ ��
��
(3)��A��D��F���Ƕ����ڷǽ���Ԫ�ص��ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ���Ӧ�ٵĻ�ѧ����ʽΪ____________________������E�ĽṹʽΪ__________

(1)��AΪ�����ڽ������ʣ�DΪ�����ڷǽ������ʣ�������Ԫ�ص�ԭ������A��D��2��������Ԫ�ص�ԭ������������D��A��2����F��Ũ��Һ��A��D��Ӧ���к���ɫ�������ɣ�
��A��ԭ�ӽṹʾ��ͼΪ__________����Ӧ�ܵĻ�ѧ����ʽΪ�� ��
(2)��AΪ�����Ľ������ʣ�D��F����̬���ʣ���Ӧ����ˮ��Һ�н��У���Ӧ��(��ˮ��Һ�н���)�����ӷ���ʽ��____________________����֪������1g D��F��Ӧ����B(��̬)ʱ�ų�92.3kJ������д����Ӧ���Ȼ�ѧ����ʽ____________________
 ��
��(3)��A��D��F���Ƕ����ڷǽ���Ԫ�ص��ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ���Ӧ�ٵĻ�ѧ����ʽΪ____________________������E�ĽṹʽΪ__________
(��12�֣�ÿ��2��)
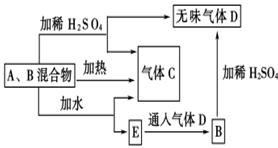
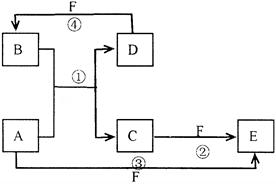
(1) �� C��4HNO3(Ũ)
�� C��4HNO3(Ũ)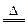 CO2����4NO2����2H2O
CO2����4NO2����2H2O
 (2)2Fe2����Cl2=2Fe3����2Cl
(2)2Fe2����Cl2=2Fe3����2Cl �� ; H2(g)��Cl2(g)
�� ; H2(g)��Cl2(g) 2HCl(g)����H=��184.6kJ��mol��1 (3)2C��SiO2
2HCl(g)����H=��184.6kJ��mol��1 (3)2C��SiO2 2CO����Si�� O=C=O
2CO����Si�� O=C=O
(1)
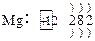 �� C��4HNO3(Ũ)
�� C��4HNO3(Ũ)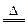 CO2����4NO2����2H2O
CO2����4NO2����2H2O (2)2Fe2����Cl2=2Fe3����2Cl
(2)2Fe2����Cl2=2Fe3����2Cl �� ; H2(g)��Cl2(g)
�� ; H2(g)��Cl2(g) 2HCl(g)����H=��184.6kJ��mol��1 (3)2C��SiO2
2HCl(g)����H=��184.6kJ��mol��1 (3)2C��SiO2 2CO����Si�� O=C=O
2CO����Si�� O=C=O��

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ
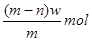 ��
��