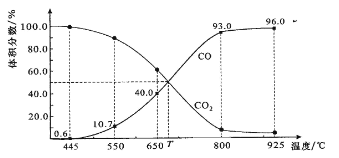
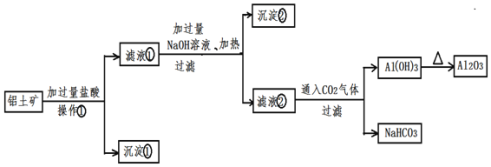
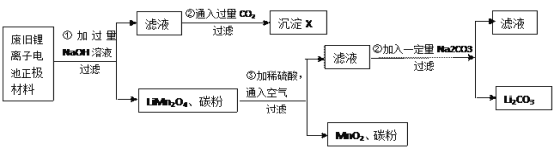
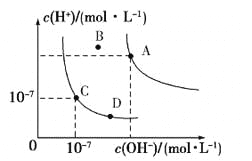
��Ŀ����
����Ŀ���Է�������NiO������Ϊ����Fe2O3������Ni2O3��һ�ֹ����������£�
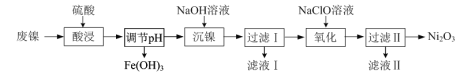
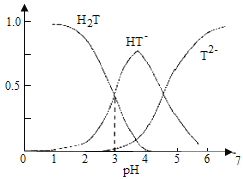
��1��������pH���������Һ�в��ٴ���Fe3���ķ�����______��
��2������������Ŀ���ǽ���Һ�е�Ni2��ת��ΪNi(OH)2������ȷ��Ni2���Ѿ���ȫ������ʵ�鷽����______��
��3������Һ��������������ҪΪCl����д����������ʱ��Ӧ�����ӷ���ʽ��______��
��4��������������Ӧ����֣�����Ni2O3��Ʒ�л����Ni(OH)2��Ϊ�ⶨ��Ʒ��Ni2O3����������������ʵ�飺��ȡ5.000 g��Ʒ����������������ټ���100 mL 1.0 mol��L��1��Fe2������Һ����ַ�Ӧ����ˮ������200 mL��ȡ��20.00 mL����0.040 mol��L��1 KMnO4����Һ�ζ�����ȥKMnO4����Һ20.00 mL����ͨ������ȷ��Ni2O3�������������ⶨ�������漰��Ӧ���£�Ni2O3��Fe2����H����Ni2����Fe3����H2O��δ��ƽ����Fe2����MnO4����H����Fe3����Mn2����H2O��δ��ƽ��______
���𰸡�ȡ����������pH����������Һ�������еμ�KSCN��Һ����Һ����� ���ã����ϲ���Һ�м����μ�NaOH��Һ���������� 2Ni(OH)2��ClO��Ni2O3��Cl��2H2O 99.24%
��������
��1������Fe3������KSCN��Һ��
��2��ȷ��Ni2���Ѿ���ȫ�����ķ���Ϊ���鷴Ӧ����ϲ���Һ���Ƿ���Ni2����
��3��Ni(OH)2��ClO-��������Ni2O3��ClO-����ԭ����Cl�������ݵ�ʧ�����غ㡢����غ㡢Ԫ���غ㣬��д����Ӧ�����ӷ���ʽ��
��4���������֪����ȡ5.000 g��Ʒ����������������ټ���100 mL 1.0 mol��L��1��Fe2������Һ����ַ�Ӧ��������ӦNi2O3��2Fe2����6H����2Ni2����2Fe3����3H2O���ټ�ˮ������200 mL��ȡ��20.00 mL����0.040 mol��L��1 KMnO4����Һ�ζ�����ȥKMnO4����Һ20.00 mL��������Ӧ5Fe2����MnO4����8H����5Fe3����Mn2����4H2O��KMnO4����Һ���ڵζ���һ����Ӧʣ���Fe2�����ݴ˽��м��㡣
��1������Fe3������KSCN��Һ�����巽��Ϊ��ȡ����������pH����������Һ�������еμ�KSCN��Һ����Һ����죬֤����Һ�в��ٴ���Fe3����
�ʴ�Ϊ��ȡ����������pH����������Һ�������еμ�KSCN��Һ����Һ����죬֤����Һ�в��ٴ���Fe3����
��2��ȷ��Ni2���Ѿ���ȫ������ʵ�鷽��Ϊ�����ã����ϲ���Һ�м����μ�NaOH��Һ���������ɣ�֤��Ni2���Ѿ���ȫ������
�ʴ�Ϊ�����ã����ϲ���Һ�м����μ�NaOH��Һ���������ɣ�֤��Ni2���Ѿ���ȫ������
��3��Ni(OH)2��ClO-��������Ni2O3��ClO-����ԭ����Cl�������ݵ�ʧ�����غ㡢����غ㡢Ԫ���غ㣬��д����Ӧ�����ӷ���ʽΪ��2Ni(OH)2��ClO��Ni2O3��Cl��2H2O��
�ʴ�Ϊ��2Ni(OH)2��ClO��Ni2O3��Cl��2H2O��
��4���������֪����ȡ5.000 g��Ʒ����������������ټ���100 mL 1.0 mol��L��1��Fe2������Һ����ַ�Ӧ��������ӦNi2O3��2Fe2����6H����2Ni2����2Fe3����3H2O���ټ�ˮ������200 mL��ȡ��20.00 mL����0.040 mol��L��1 KMnO4����Һ�ζ�����ȥKMnO4����Һ20.00 mL��������Ӧ5Fe2����MnO4����8H����5Fe3����Mn2����4H2O��KMnO4����Һ���ڵζ���һ����Ӧʣ���Fe2������������KMnO4����Һ�����ʵ�������ʣ��Fe2�����ʵ�������������¹�ϵʽ�ɵ�
5Fe2�� ~ MnO4��
0.004mol 0.040 mol��L��1��0.02L
����Ni2O3��Ӧʣ��Fe2�����ʵ���Ϊ0.004mol��10=0.04mol����������¹�ϵʽ����Ni2O3�����ʶ�����
Ni2O3 ~ 2Fe2��
0.03mol 0.1L��1.0 mol��L��1-0.04mol=0.06mol
����Ʒ��Ni2O3����������Ϊ![]() ��
��
�ʴ�Ϊ��99.24%��

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�