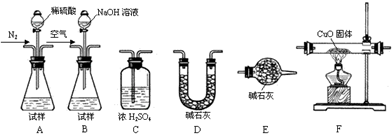
��Ŀ����
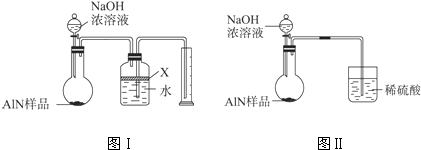
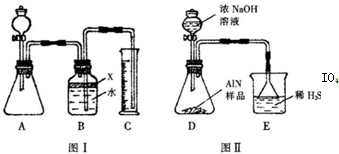
��������AlN����һ�����������ϣ��㷺Ӧ���뼯�ɵ�·��������ij�������к���̼�����������ʣ�����ͼ���е�һЩװ�������м��飬ʹ��������Ʒ��NaOH��Һ��ӦAlN+NaOH+H2O=NaAlO2+NH3�������ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ���

��1��ʵ���йز���Ϊ��a������ƿ�з���������AlN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH��c������װ�õ������ԣ�d���ⶨ�ռ���ˮ���������ȷ�IJ���˳��Ϊ______��
��2���������м��װ�������Եķ�����______��
��3�����ƿ�е��Լ�X��ѡ��______����ѡ��ı�ţ���
A��Ũ����������Һ����B���ƾ�������C��ֲ���͡�����D��CCl4
��4�����ƿ��Һ��δװ�������NH3�������______���ƫ����ƫС������Ӱ�족����
��5��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������______��
��6������Ʒ������Ϊw g�����������Ϊa L����״������AlN����������Ϊ______��
�⣺��1��Ӧ�Ƚ���װ�������Լ��飬Ȼ�����μ������ҩƷ��Һ��ҩƷ�������������ų�ˮ�IJ�����ȷ���������������
�ʴ�Ϊ��c��a��b��d��
��2��ͨ���Ȼ���������ʹװ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣻
�ʴ�Ϊ���رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժã�
��3��Ũ����������Һ��ˮ���ܣ������������ã��ƾ��ӷ����ӷ������������ʵ����Ӱ�죬ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ�CCl4�ܶȴ���ˮ�������������ã���ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ����ڣ�����Բ����������Ӱ�죻
�ʴ�Ϊ����Ӱ�죻
��5���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ������������̼��
�ʴ�Ϊ��̼��
��6�����������ΪaL������£������ʵ���Ϊ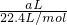 =
=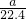 mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=
mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=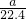 mol������AlN������Ϊ
mol������AlN������Ϊ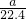 mol��41g/mol=
mol��41g/mol=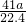 g����Ʒ��AIN����������Ϊ
g����Ʒ��AIN����������Ϊ ��100%=
��100%=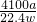 %��
%��
�ʴ�Ϊ��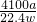 %��
%��
��������1����ȡ����ʱ��Ϊ��ֹװ��©��Ӧ������װ�ú���������װ�������Լ�飬ȷ��װ�ò�©�������ȼӹ�����Һ���ԭ�����ҩƷ��������������ռ��������
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��ѡ�õ��Լ�Ӧ�Ǻ�ˮ�����ܣ����ܶȴ���ˮ�ģ�
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ�����Χ�ڣ�
��5���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ��
��6����������������ΪaL�����ʵ������ٸ��ݷ���ʽ�����AlN�����ʵ�������������AlN���������������������Ķ��������Ʒ��AIN������������
���������⿼���ʵ��ԭ����������ʵ��������ۡ����ʺ����ⶨ����ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ��
�ʴ�Ϊ��c��a��b��d��
��2��ͨ���Ȼ���������ʹװ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣻
�ʴ�Ϊ���رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժã�
��3��Ũ����������Һ��ˮ���ܣ������������ã��ƾ��ӷ����ӷ������������ʵ����Ӱ�죬ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ�CCl4�ܶȴ���ˮ�������������ã���ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ����ڣ�����Բ����������Ӱ�죻
�ʴ�Ϊ����Ӱ�죻
��5���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ������������̼��
�ʴ�Ϊ��̼��
��6�����������ΪaL������£������ʵ���Ϊ
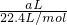 =
=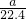 mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=
mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=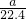 mol������AlN������Ϊ
mol������AlN������Ϊ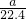 mol��41g/mol=
mol��41g/mol=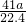 g����Ʒ��AIN����������Ϊ
g����Ʒ��AIN����������Ϊ ��100%=
��100%=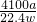 %��
%���ʴ�Ϊ��
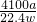 %��
%����������1����ȡ����ʱ��Ϊ��ֹװ��©��Ӧ������װ�ú���������װ�������Լ�飬ȷ��װ�ò�©�������ȼӹ�����Һ���ԭ�����ҩƷ��������������ռ��������
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��ѡ�õ��Լ�Ӧ�Ǻ�ˮ�����ܣ����ܶȴ���ˮ�ģ�
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ�����Χ�ڣ�
��5���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ��
��6����������������ΪaL�����ʵ������ٸ��ݷ���ʽ�����AlN�����ʵ�������������AlN���������������������Ķ��������Ʒ��AIN������������
���������⿼���ʵ��ԭ����������ʵ��������ۡ����ʺ����ⶨ����ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ��

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�
�����Ŀ