��Ŀ����
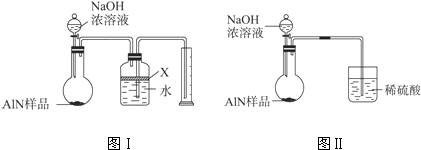
��������AlN����һ�����͵������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij��������Ʒ�к���̼�����������ʣ�����ͼIװ�ý���ʵ�飬ʹ��������Ʒ��NaOH��Һ��Ӧ����Ӧ�Ļ�ѧ����ʽΪAlN+NaOH+H2O�TNaAlO2+NH3���������ݷ�Ӧ�����ɵİ�����������ⶨ��Ʒ�е�����������������������ʵ������ȷ�����ʵijɷ֣�ʵ����̼�¼���£�

a�����װ�õ������ԣ�
b����ͼIװ�õ���ƿ�з���������������Ʒ���ӷ�Һ©������ƿ�м������ŨNaOH��Һ�������������ҷ�Ӧ�����ɵİ������ƿ�е�ˮ������Ͳ�У�
c��ʵ�����������Ͳ���ռ�����ˮ������������ɹ��ƿ����Ͳ�ĵ����ڵ�ˮ��������������м��㣮
��1�����Aװ�������Եķ�����
��2��Bװ�ù��ƿ�е��Լ�X��ѡ�� ��������ѡ����ĸ��
A������ B���ƾ� C��ֲ���� D�����Ȼ�̼
��3��Bװ�ù��ƿ�е�Һ��û��װ�����Ϸ����������ռ䣩��ʵ������õ�NH3������� �����ƫ����ƫС������Ӱ�족����
��4��ʵ����������۲쵽Aװ����ƿ�л��й��壬����Ʒ�к��е������� ��
��5������ȡwg��Ʒ����ʵ�飬ʵ�����ʱ��ð��������ΪaL��������ɱ�״����������Ʒ��AlN����������Ϊ ���ú�w��a�Ĺ�ϵʽ��ʾ�����û���
��6�����˸�����ͼDװ�ý���ͬ��ʵ�飬ͨ���ⶨEװ�����ӵ�������ȷ����Ʒ��AlN����������������Ϊ����ʵ�鷽���� ���ã��ͼI����ͼ���������� ��

a�����װ�õ������ԣ�
b����ͼIװ�õ���ƿ�з���������������Ʒ���ӷ�Һ©������ƿ�м������ŨNaOH��Һ�������������ҷ�Ӧ�����ɵİ������ƿ�е�ˮ������Ͳ�У�
c��ʵ�����������Ͳ���ռ�����ˮ������������ɹ��ƿ����Ͳ�ĵ����ڵ�ˮ��������������м��㣮
��1�����Aװ�������Եķ�����
��2��Bװ�ù��ƿ�е��Լ�X��ѡ��
A������ B���ƾ� C��ֲ���� D�����Ȼ�̼
��3��Bװ�ù��ƿ�е�Һ��û��װ�����Ϸ����������ռ䣩��ʵ������õ�NH3�������
��4��ʵ����������۲쵽Aװ����ƿ�л��й��壬����Ʒ�к��е�������
��5������ȡwg��Ʒ����ʵ�飬ʵ�����ʱ��ð��������ΪaL��������ɱ�״����������Ʒ��AlN����������Ϊ
��6�����˸�����ͼDװ�ý���ͬ��ʵ�飬ͨ���ⶨEװ�����ӵ�������ȷ����Ʒ��AlN����������������Ϊ����ʵ�鷽����
��������1��ֻҪ�Ƚ�װ���ܷ���������������ԭ��������������֤��
��2�����ݹ��ƿ�е��Լ�X�����ÿ��ǣ�
��3�����ݷ�Ӧǰ����ƿ���Ϸ����е������ռ��������仯���ǣ�
��4�����������Ƿ�������������Һ��Ӧ���ǣ�
��5���ȸ���ÿ22.4L��������Ϊ17g����������������ΪaL���������ٸ��ݷ���ʽ�����AlN���������ٳ���wg���ٷ�֮�٣�
��6�����ݰ������������ᣬ������ȫ���������գ�ȷ�ⶨ�����������ʵ��ɰܵĹؼ����ش�
��2�����ݹ��ƿ�е��Լ�X�����ÿ��ǣ�
��3�����ݷ�Ӧǰ����ƿ���Ϸ����е������ռ��������仯���ǣ�
��4�����������Ƿ�������������Һ��Ӧ���ǣ�
��5���ȸ���ÿ22.4L��������Ϊ17g����������������ΪaL���������ٸ��ݷ���ʽ�����AlN���������ٳ���wg���ٷ�֮�٣�
��6�����ݰ������������ᣬ������ȫ���������գ�ȷ�ⶨ�����������ʵ��ɰܵĹؼ����ش�
����⣺��1��������ѹԭ��������װ�������Եķ����ǣ��رշ�Һ©�����أ�ʹװ�ô����ܱ���ϵ��������һ�˽���ˮ�У����ֽ�����ƿ��ڣ�����������������ƿ�������������ͣ�������ܿ�������ð����˵�����������ã�����װ��©����
�ʴ�Ϊ���رշ�Һ©�����أ�ʹװ�ô����ܱ���ϵ��������һ�˽���ˮ�У����ֽ�����ƿ��ڣ�����������������ƿ�������������ͣ�������ܿ�������ð����˵�����������ã�����װ��©����
��2�����ƿ�е��Լ�X�������ǽ�������ˮ��������Ϊ������������ˮ�����ھƾ��������������ˮ���ܣ����Բ����þƾ���Ҫ��ֲ���ͽ�������ˮ������ˮ�������ͣ�
�ʴ�Ϊ��C��
��3����Ӧǰ���ƿ���Ϸ����е������ռ������ǿ�������Ӧ����ƿ���Ϸ����е������ռ������ǰ��������������˿�ʼ�Ŀ����������ռ��г���İ������ɿ�ʼʱ�Ŀ������ɣ����Զ�ʵ����û��Ӱ�죬�ʴ�Ϊ����Ӱ�죻
��4���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ������������̼��
�ʴ�Ϊ��̼��
��5������ÿ22.4L��������Ϊ17g����������������ΪaL��������
����������Щ��������Ҫ�μӷ�Ӧ�ĵ���������ΪX��
AlN+NaOH+H2O=NaAlO2+NH3��
41 17
X
���ݣ�
=
���X=
��������Ʒ��AlN��������Ϊ��
��100%=
��100%���ʴ�Ϊ��
��100%��
��6��ͼ��װ�ÿ���ȷ�IJⶨ�����������ȷ���㵪�����İٷֺ��������ǰ���װ�â�������飬��Ӧ���ɵİ���������ȫ���������գ������ᵼ��ʵ��ȷ�Բ�ʴ�Ϊ��ͼ���������װ�â�������飬��Ӧ���ɵİ���������ȫ���������գ�ʵ��ȷ�Բ
�ʴ�Ϊ���رշ�Һ©�����أ�ʹװ�ô����ܱ���ϵ��������һ�˽���ˮ�У����ֽ�����ƿ��ڣ�����������������ƿ�������������ͣ�������ܿ�������ð����˵�����������ã�����װ��©����
��2�����ƿ�е��Լ�X�������ǽ�������ˮ��������Ϊ������������ˮ�����ھƾ��������������ˮ���ܣ����Բ����þƾ���Ҫ��ֲ���ͽ�������ˮ������ˮ�������ͣ�
�ʴ�Ϊ��C��
��3����Ӧǰ���ƿ���Ϸ����е������ռ������ǿ�������Ӧ����ƿ���Ϸ����е������ռ������ǰ��������������˿�ʼ�Ŀ����������ռ��г���İ������ɿ�ʼʱ�Ŀ������ɣ����Զ�ʵ����û��Ӱ�죬�ʴ�Ϊ����Ӱ�죻
��4���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ������������̼��
�ʴ�Ϊ��̼��
��5������ÿ22.4L��������Ϊ17g����������������ΪaL��������
| 17a |
| 22.4L/mol |
AlN+NaOH+H2O=NaAlO2+NH3��
41 17
X
| 17a |
| 22.4 |
���ݣ�
| 41 |
| 17 |
| X | ||
|
| 41a |
| 22.4 |
| 41a |
| 22.4w |
| 41a |
| 22.4w |
| 41a |
| 22.4w |
��6��ͼ��װ�ÿ���ȷ�IJⶨ�����������ȷ���㵪�����İٷֺ��������ǰ���װ�â�������飬��Ӧ���ɵİ���������ȫ���������գ������ᵼ��ʵ��ȷ�Բ�ʴ�Ϊ��ͼ���������װ�â�������飬��Ӧ���ɵİ���������ȫ���������գ�ʵ��ȷ�Բ
�����������Ҫ֪����ȡ�����һ�㲽�裬֪������ˮ���ⶨ��������ķ��������ڰ�����������ˮ��Ҫ��ֲ���ͽ�������ˮ�ֿ���֪�����뷽��ʽ�������ֵֻ����������������������������ٽ����б���ʽ���㣮

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ