��Ŀ����
��2011?����ģ�⣩��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�����ͼI�е�һЩװ�������м��飬ʹ��������Ʒ��NaOH��Һ��Ӧ��
AlN+NaOH+H2O�TNaAlO2+NH3��
���ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�

��1��ʵ���йز���Ϊ��a������ƿ�з���������AIN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH�� C������װ�õ������ԣ�d���ⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ��
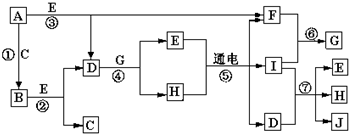
��2���������У�ͼI�����װ�������Եķ����ǣ�
��3�����ƿ�е��Լ�X��ѡ��
A������ B���ƾ� C��ֲ���� D��CCl4
��4��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������
��5����ʵ���в����Ʒ������Ϊw g�����������ΪaL������£�������Ʒ��AIN����������Ϊ��
%
%��
��6�����˸���ͼIIװ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AIN����������������Ϊ�Ƿ���У�
AlN+NaOH+H2O�TNaAlO2+NH3��
���ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�

��1��ʵ���йز���Ϊ��a������ƿ�з���������AIN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH�� C������װ�õ������ԣ�d���ⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ��
c��a��b��d
c��a��b��d
����2���������У�ͼI�����װ�������Եķ����ǣ�
�رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժ�
�رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժ�
����3�����ƿ�е��Լ�X��ѡ��
C
C
������ѡ��ı�ţ�A������ B���ƾ� C��ֲ���� D��CCl4
��4��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������
̼
̼
����5����ʵ���в����Ʒ������Ϊw g�����������ΪaL������£�������Ʒ��AIN����������Ϊ��
| 4100a |
| 22.4w |
| 4100a |
| 22.4w |
��6�����˸���ͼIIװ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AIN����������������Ϊ�Ƿ���У�
������
������
�����롰���С����������С�����ԭ�����������ױ����գ�������������
�������ױ����գ�������������
����������1����ȡ����ʱ��Ϊ��ֹװ��©��Ӧ������װ�ú���������װ�������Լ�飬ȷ��װ�ò�©�������ȼӹ�����Һ���ԭ�����ҩƷ��������������ռ��������
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��ѡ�õ��Լ�Ӧ�Ǻ�ˮ�����ܣ����ܶȴ���ˮ�ģ�
��4���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ��
��5����������������ΪaL�����ʵ������ٸ��ݷ���ʽ�����AlN�����ʵ�������������AlN���������������������Ķ��������Ʒ��AIN������������
��6�������������ᷴӦ��������臨�ʹϡ������Һ�������ӣ������ڷ�Ӧ��Ϊ���Ҷ���ʹϡ���ᵹ����������ձ���������ȷ��Ϊ�����������֣����ڵ���ĩ�˰�װ����©����ֹ������
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��ѡ�õ��Լ�Ӧ�Ǻ�ˮ�����ܣ����ܶȴ���ˮ�ģ�
��4���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ��
��5����������������ΪaL�����ʵ������ٸ��ݷ���ʽ�����AlN�����ʵ�������������AlN���������������������Ķ��������Ʒ��AIN������������
��6�������������ᷴӦ��������臨�ʹϡ������Һ�������ӣ������ڷ�Ӧ��Ϊ���Ҷ���ʹϡ���ᵹ����������ձ���������ȷ��Ϊ�����������֣����ڵ���ĩ�˰�װ����©����ֹ������
����⣺����1��Ӧ�Ƚ���װ�������Լ��飬Ȼ�����μ������ҩƷ��Һ��ҩƷ�������������ų�ˮ�IJ�����ȷ���������������
�ʴ�Ϊ��c��a��b��d��
��2��ͨ���Ȼ���������ʹװ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣻
�ʴ�Ϊ���رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժã�
��3���ƾ���������Ȼ�������백��������Ӧ��������ȴ�����ӷ����ӷ������������ʵ����Ӱ����һӷ�����������백����ˮ�Ӵ������ã�ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ�CCl4�ܶȴ���ˮ�������������ã���ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ������������̼��
�ʴ�Ϊ��̼��
��5�����������ΪaL������£������ʵ���Ϊ
=
mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=
mol������AlN������Ϊ
mol��41g/mol=
g����Ʒ��AIN����������Ϊ
��100%=
%��
�ʴ�Ϊ��
%��
��6��������������ϡ��������ֵ�������ˣ���װ�ò���ȷ���������������������ڵ���ĩ������©��������Һ���ϣ��հ�����������ʱ���ձ���Һ���½�������Ӵ������Է�ֹϡ����ĵ�����
�ʴ�Ϊ�������У��������ױ����գ�������������
�ʴ�Ϊ��c��a��b��d��
��2��ͨ���Ȼ���������ʹװ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣻
�ʴ�Ϊ���رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժã�
��3���ƾ���������Ȼ�������백��������Ӧ��������ȴ�����ӷ����ӷ������������ʵ����Ӱ����һӷ�����������백����ˮ�Ӵ������ã�ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ�CCl4�ܶȴ���ˮ�������������ã���ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4���������к���̼�����������ʣ�������������NaOH��Һ����ʵ����������۲쵽��ƿ�л��й��壬˵�����岻��������������Һ������������̼��
�ʴ�Ϊ��̼��
��5�����������ΪaL������£������ʵ���Ϊ
| aL |
| 22.4L/mol |
| a |
| 22.4 |
| a |
| 22.4 |
| a |
| 22.4 |
| 41a |
| 22.4 |
| ||
| wg |
| 4100a |
| 22.4w |
�ʴ�Ϊ��
| 4100a |
| 22.4w |
��6��������������ϡ��������ֵ�������ˣ���װ�ò���ȷ���������������������ڵ���ĩ������©��������Һ���ϣ��հ�����������ʱ���ձ���Һ���½�������Ӵ������Է�ֹϡ����ĵ�����
�ʴ�Ϊ�������У��������ױ����գ�������������
���������⿼���ʵ��ԭ����������ʵ��������ۡ����ʺ����ⶨ����ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ