��Ŀ����
K��Ki��KW�ֱ��ʾ��ѧƽ�ⳣ�������볣����ˮ�����ӻ��������ж���ȷ����
| A����500�桢20MPa��5L���ܱ������н��кϳɰ��ķ�Ӧ��ʹ�ô�����Kֵ���� |
| B��������K(HCN)��K(CH3COOH)��˵��CH3COOH�ĵ����һ����HCN�� |
| C��25��ʱ��pH ��Ϊ4�������NH4I(aq)��KW����� |
D��2SO2+O2 2SO3��ƽ��ı�ijһ����Kֵ���䣬SO2��ת���ʿ�������С�� 2SO3��ƽ��ı�ijһ����Kֵ���䣬SO2��ת���ʿ�������С�� |
D
���������A����ѧƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��仯ѧƽ�ⳣ�����䣬ʹ�ô����ӿ췴Ӧ���ʣ�ƽ�ⲻ�ƶ�����ѧƽ�ⳣ�����䣬��A����B����ͬ�¶���CH3COOH��HCN�ĵ��������ʼŨ�ȡ�ͬ����ЧӦ���йأ���B����C��ˮ�����ӻ��������ᡢ�����Һ��һ���¶��£�ˮ�����ӻ��dz�����25��ʱ�������NH4I��aq����KW��ȣ���C����D���ı�ѹǿƽ�ⷢ���ƶ���SO2��ת���ʿ�������С��ʹ�ô���ƽ�ⲻ�ƶ�����D��ȷ����ѡD��

��ϰ��ϵ�д�
�����Ŀ
 p C��ij�¶��´ﵽƽ��
p C��ij�¶��´ﵽƽ�� CH3OH��g��
CH3OH��g��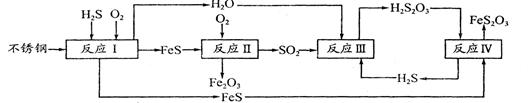
 H2S(g) ��H=��21��6kJ��mol��1����Ӧ�ﵽƽ��ʱH2��S��H2S�����ʵ�����Ϊ3 mol����380 Kʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ______�����жԸ÷�Ӧ������ȷ����______(����ĸ���)��
H2S(g) ��H=��21��6kJ��mol��1����Ӧ�ﵽƽ��ʱH2��S��H2S�����ʵ�����Ϊ3 mol����380 Kʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ______�����жԸ÷�Ӧ������ȷ����______(����ĸ���)��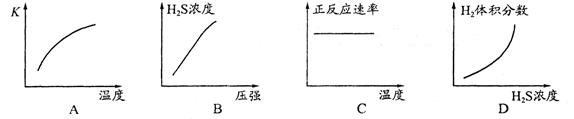
 C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
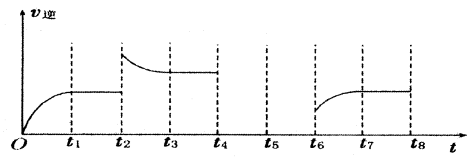
C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£� B(g)+C(g)����H ="-48.25" kJ �� mol��1��Ӧ������ʱ��t��A ��BŨ������ͼ��ʾ��ϵ������õ�15minʱc(B)="1.6" mol��L��1�������н�����ȷ����
B(g)+C(g)����H ="-48.25" kJ �� mol��1��Ӧ������ʱ��t��A ��BŨ������ͼ��ʾ��ϵ������õ�15minʱc(B)="1.6" mol��L��1�������н�����ȷ����
 xC(g) ��H��0�������������c(A)��ʱ��t�ı仯����ͼ��ʾ������˵������ȷ����
xC(g) ��H��0�������������c(A)��ʱ��t�ı仯����ͼ��ʾ������˵������ȷ����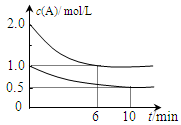
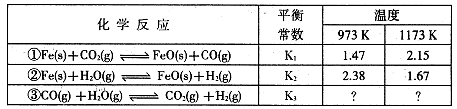
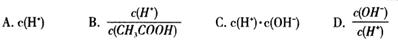
 H2 (g) +CO2 (g)ƽ�ⳣ��K���¶ȵı仯���±���
H2 (g) +CO2 (g)ƽ�ⳣ��K���¶ȵı仯���±���
 2CO (g)ƽ�ⳣ��K1��
2CO (g)ƽ�ⳣ��K1�� CO(g) +H2 (g)ƽ�ⳣ��K2��K��K1��K2,֮��Ĺ�ϵ�� :
CO(g) +H2 (g)ƽ�ⳣ��K2��K��K1��K2,֮��Ĺ�ϵ�� :