��Ŀ����
����Ŀ��80 ��ʱ����0.4 mol�������������������2 L�ѳ�յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
ʱ��/s c(mol��L��1) | 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4) | 0.20 | a | 0.10 | c | d | e |
c(NO2) | 0.00 | 0.12 | b | 0.22 | 0.22 | 0.22 |
��Ӧ������100 s��Ӧ�������¶Ƚ��ͣ������������ɫ��dz��
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ______________________________________________��
(2)����b��______��c______d(����������������������)��
(3)20 sʱ��N2O4��Ũ��Ϊ______mol��L��1��0��20 s��N2O4��ƽ����Ӧ����Ϊ________��
(4)�÷�Ӧ��ƽ�ⳣ������ʽK��_____����80 ��ʱ�÷�Ӧ��ƽ�ⳣ��KֵΪ________��
(5)������������ͬʱ���÷�Ӧ��KֵԽ��������ƽ��ʱ________��
A��N2O4��ת����Խ�� B��NO2�IJ���Խ��
C��N2O4��NO2��Ũ��֮��Խ�� D������Ӧ���еij̶�Խ��
E������ʱ��Խ�� F������������ѹǿԽ��
���𰸡�N2O4![]() 2NO2 b=0.2mol/L = 0.14 0.003mol��L��1��s��1 K=
2NO2 b=0.2mol/L = 0.14 0.003mol��L��1��s��1 K= 0.54 ABDF
0.54 ABDF
��������
(1)����ͼ�����ݷ�������Ӧ�������������жϣ�
(2)40sʱN2O4�ı仯Ũ��Ϊ0.10molL-1����NO2�ı仯Ũ��Ϊ0.20molL-1��60s��Ӧ�ﵽƽ��״̬����Ӧ������������������ʱ��ı仯���仯��
(3)��ѧƽ�������ʽ��ʽ���㣬��ϻ�ѧ��Ӧ���ʸ������0��20s��N2O4��ƽ����Ӧ���ʣ�
(4)���ݻ�ѧ����ʽ��ƽ�ⳣ������д��ƽ�ⳣ������ʽ������80��Cƽ��״̬�½������ʽ��ʽ����ƽ��Ũ�ȼ���ƽ�ⳣ����
(5)����ƽ�ⳣ���Ķ����֪��KֵԽ��������Ũ��Խ��Ӧ��Ũ��ԽС����Ӧ������еij̶�Խ�ݴ˴��⡣
(1)��0.4mol�������������������2L�ѳ�յĹ̶��ݻ����ܱ������У���Ӧ�Ļ�ѧ����ʽΪN2O42NO2��
(2)40sʱN2O4�ı仯Ũ��Ϊ0.10molL-1����NO2�ı仯Ũ��Ϊ0.20molL-1����b��ֵΪ0.20molL-1��60s��Ӧ�ﵽƽ��״̬����Ӧ������������������ʱ��ı仯���仯����c=d��
(3)���е�20S��
N2O4 2NO2
��ʼ��(mol) 0.4 0
�仯��(mol) 0.12 0.24
20Sĩ(mol) 0.28 0.24
20sʱ��N2O4��Ũ��=![]() =0.14mol/L��0��20s��N2O4��ƽ����Ӧ����=
=0.14mol/L��0��20s��N2O4��ƽ����Ӧ����=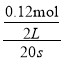 =0.003mol��L��1��s��1��
=0.003mol��L��1��s��1��
(4)N2O42NO2��ƽ�ⳣ��K= ��
��
80��Cƽ��״̬���Ƿ�Ӧ�ﵽ60Sʱ��״̬��ƽ��Ũ��c(NO2)=0.22mol/L��c(N2O4)=0.09mol/L��ƽ�ⳣ��K= =
=![]() =0.54��
=0.54��
(5)����ƽ�ⳣ���Ķ����֪��KֵԽ��������Ũ��Խ��Ӧ��Ũ��ԽС����Ӧ������еij̶�Խ��
A����Ӧ������У�KֵԽ��N2O4��ת����Խ�ߣ���A��ȷ��
B����Ӧ������У�KֵԽ��NO2�IJ���Խ��B��ȷ��
C��KֵԽ��������Ũ��Խ��Ӧ��Ũ��ԽС��N2O4��NO2��Ũ��֮��ԽС����C����
D��KֵԽ��������Ũ��Խ��Ӧ��Ũ��ԽС����Ӧ������еij̶�Խ��D��ȷ��
E��ƽ�ⳣ����ﵽƽ�������ĵ�ʱ���ޱ�Ȼ��ϵ����E����
F�����º��������£�����������ѹǿԽ��˵�����ɵ�NO2�IJ���Խ����KֵԽ��F��ȷ��
�ʴ�ΪABDF��

 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�����Ŀ������ʵ����ʵ����Ӧ��������ȷ����
ѡ�� | ʵ����ʵ | ���� |
A | �����������䣬0.01 mol��L��1������KMnO4��Һ�ֱ���0.1 mol��L��1��H2C2O4��Һ��0.2 mol��L��1��H2C2O4��Һ��Ӧ��������ɫʱ��� | ��������������ʱ������Ӧ��Ũ�ȿ���ʹ��ѧ��Ӧ���ʼӿ� |
B | �����������䣬�ֱ�����������ʵ���Ũ�ȵ�Na2S2O3��Һ��H2SO4���Һ������ˮ����ˮ�У�������ˮ�еĻ��Һ�ȳ��ֻ��� | ��������������ʱ����Ӧ��ϵ���¶�Խ�ߣ���ѧ��Ӧ����Խ�� |
C | ������MnO2��ĩ����ʢ��10%˫��ˮ����ƿ�ڣ��ڻ�ѧ��Ӧǰ��MnO2�������ͻ�ѧ���ʶ�û�з����ı� | ������Ȼ���Լӿ컯ѧ��Ӧ�����ʣ���һ�������뻯ѧ��Ӧ���� |
D | һ�������£��ֱ����ݻ�Ϊ1 L���ݻ�Ϊ2 L�������ܱ������м�������������͵��������������·�Ӧ��H2(g)��I2(g) | ��������������ʱ����̬��Ӧ��ϵ��ѹǿԽ��ѧ��Ӧ����Խ�� |
A. A B. B C. C D. D


