��Ŀ����
��8�֣��������γɶ������ӣ���N3����NH2����N3����NH4+��N2H5+��N2H62+�ȣ���֪N2H5+��N2H62+�������Է��ӽ�������γɵģ�������NH4+������������� NH4+�����ʡ�
��1��NH4+�ĵ���ʽΪ ��N���� �ӻ���ʽ��NH4+���ӿռ乹��Ϊ ������Ϊ ��
��2��д�������ɶ��ԭ����ɵĺ�����N3���۵�������ͬ�����ʵĻ�ѧʽ ��
��3���ȵ�������������������ƵĽṹ����Ԥ��N3���Ĺ��� ��
��1��NH4+�ĵ���ʽΪ ��N���� �ӻ���ʽ��NH4+���ӿռ乹��Ϊ ������Ϊ ��
��2��д�������ɶ��ԭ����ɵĺ�����N3���۵�������ͬ�����ʵĻ�ѧʽ ��
��3���ȵ�������������������ƵĽṹ����Ԥ��N3���Ĺ��� ��
��1��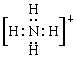 sp3 ������ 109��28�� (2) CO2��N2O ��3��ֱ����
sp3 ������ 109��28�� (2) CO2��N2O ��3��ֱ����
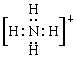 sp3 ������ 109��28�� (2) CO2��N2O ��3��ֱ����
sp3 ������ 109��28�� (2) CO2��N2O ��3��ֱ������1��NH4+�к��й��ۼ������Ե���ʽΪ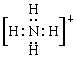 �����ڸ����������������νṹ���������109��28�䣬���Ե�ԭ����sp3�ӻ���
�����ڸ����������������νṹ���������109��28�䣬���Ե�ԭ����sp3�ӻ���
��2��N3���۵�������5��3��1��16����������N3���۵�������ͬ�����ʵĻ�ѧʽ�� CO2��N2O��
��3������N3����CO2��Ϊ�ȵ����壬��CO2��ֱ���ͽṹ������N3���Ĺ���Ҳ��ֱ���ͽṹ��
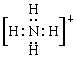 �����ڸ����������������νṹ���������109��28�䣬���Ե�ԭ����sp3�ӻ���
�����ڸ����������������νṹ���������109��28�䣬���Ե�ԭ����sp3�ӻ�����2��N3���۵�������5��3��1��16����������N3���۵�������ͬ�����ʵĻ�ѧʽ�� CO2��N2O��
��3������N3����CO2��Ϊ�ȵ����壬��CO2��ֱ���ͽṹ������N3���Ĺ���Ҳ��ֱ���ͽṹ��

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
 ����CH��CH ��NH3����CH4
����CH��CH ��NH3����CH4