��Ŀ����
10���״����о���Ϊ���������ȵ㣮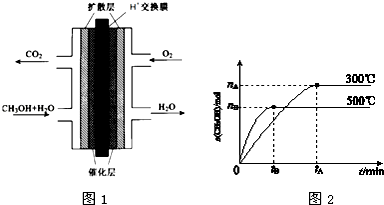
�״�ȼ�ϵ�أ�DNFC������Ϊ��21���͵綯������Ѻ�ѡ����Դ��
��1��101kP ʱ��1mol CH3OH��ȫȼ�������ȶ���������ų�����726.51kJ/mol����״�ȼ�յ��Ȼ�ѧ����ʽΪCH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-726.51kJ/mol��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
��CH3OH��g��+H2O��g��=CO2��g��+3H2��g����H1=+49.0kJ•mol-1
��CH3OH��g��+$\frac{1}{2}$O2��g��=CO2��g��+2H2��g����H2
��֪H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8kJ•mol-1��Ӧ�ڵġ�H2=-192.8kJ•mol-1��
��3���״�ȼ�ϵ�صĽṹʾ��ͼ��ͼ1�����������ĵ缫��ӦΪCH3OH+H2O-6e-�T6H++CO2��
��һ�������£������Ϊ3L���ܱ������з�ӦCO��g��+2H2��g��?CH3OH��g���ﵽ��ѧƽ��״̬��
��1������ͼ2��������ΪCH3OH�����ʵ����������¶ȣ�Kֵ����С�����������С�����䡱����
��2��500��ʱ���ӷ�Ӧ��ʼ���ﵽ��ѧƽ�⣬��H2��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������$\frac{2{n}_{B}}{3{t}_{B}}$����nB��tB��ʾ����
��3���жϸÿ��淴Ӧ�ﵽ��ѧƽ��״̬�ı�־��ac������ĸ����
a��CO��H2��CH3OH��Ũ�Ⱦ����ٱ仯 b�����������ܶȲ��ٸı�
c����������ƽ����Է����������ٸı� d��v������CH3OH��=v������CO��
e���������n��CO����n��H2����n��CH3OH��=1��2��1
��4��300��ʱ�����������ݻ�ѹ����ԭ����$\frac{1}{2}$���������������������£���ƽ����ϵ������Ӱ����cd������ĸ����
a��c��H2������
b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c��CH3OH �����ʵ�������
d������ƽ��ʱ$\frac{c��{H}_{2}��}{c��C{H}_{3}OH��}$��С��
���� ��1��1molCH3OH��ȫȼ�������ȶ���������Ϊ��̬������̼��Һ̬ˮ���ų�����726.51kJ/mol���Դ���д�Ȼ�ѧ����ʽ��
��2���ɸ�˹���ɿ�֪����CH3OH��g��+H2O��g��=CO2��g��+3H2��g����H1=+49.0kJ•mol-1����H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-241.8kJ•mol-1����+�۵õ���Ӧ�ڣ�
��3���״�ȼ�ϵ�صĸ�������ȼ�Ϸ���ʧ���ӵ�������Ӧ�����ݵ���ʻ���ȷ���缫��Ӧʽ��
��1�����������¶��Ҵ������ʵ�����С��ƽ�������ƶ����ж�ƽ�ⳣ���ı仯��
��2�����ݻ�ѧ��Ӧ�����Ǧԣ�H2��=2�ԣ�CH3OH�����㣻
��3���ﵽƽ��ʱ�����淴Ӧ������ȣ���������ƽ����Է����������ٸı䣬CO��H2��CH3OH��Ũ�Ȳ��ٸı䣻
��4�����������ݻ�ѹ����ԭ����$\frac{1}{2}$��ѹǿ����Ӧ��������ƽ��������Ӧ�����ƶ����Դ��жϣ�
��� �⣺��1��1molCH3OH��ȫȼ�������ȶ���������Ϊ��̬������̼��Һ̬ˮ���ų�����726.51kJ/mol����ȼ�յ��Ȼ�ѧ����ʽΪCH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-726.51kJ/mol��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-726.51kJ/mol��
��2���ɸ�˹���ɿ�֪����CH3OH��g��+H2O��g��=CO2��g��+3H2��g����H1=+49.0kJ•mol-1����H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-241.8kJ•mol-1����+�۵õ���Ӧ�ڣ�
���H2=+49.0kJ•mol-1+��-241.8kJ•mol-1��=-192.8kJ•mol-1���ʴ�Ϊ��-192.8kJ•mol-1��
��3���״�ȼ�ϵ�صĸ�������ȼ�Ϸ���ʧ���ӵ�������Ӧ���缫��ӦʽΪ��CH3OH+H2O-6e-�T6H++CO2���ʴ�Ϊ��CH3OH+H2O-6e-�T6H++CO2��
��1����ͼ���֪�����¶��Ҵ������ʵ������٣�ƽ�����淴Ӧ�����ƶ���K��С��
�ʴ�Ϊ����С��
��2���ԣ�CH3OH��=$\frac{nB}{nt��3L}$mol/��L��min�����ʦԣ�H2��=2�ԣ�CH3OH��=$\frac{2{n}_{B}}{3{t}_{B}}$���ʴ�Ϊ��$\frac{2{n}_{B}}{3{t}_{B}}$��
��3���ﵽƽ��ʱ�����淴Ӧ������ȣ���������ƽ����Է����������ٸı䣬CO��H2��CH3OH��Ũ�Ȳ��ٸı䣬���������ʱ��������ܶȲ��䣬������Ϊ�ж��Ƿ�ﵽƽ��״̬�����ݣ�
�ʴ�Ϊ��ac��
��4�����������ݻ�ѹ����ԭ����$\frac{1}{2}$��ѹǿ�������淴Ӧ���ʶ�����ƽ��������Ӧ�����ƶ���CH3OH�����ʵ������ӣ����������ʵ������٣���Ũ�����״������ʵ������࣬����c��H2��/c��CH3OH����С��
�ʴ�Ϊ��cd��
���� ���⿼�黯ѧƽ���Ӱ�����غͻ�ѧƽ��ı�־�����⣬��Ŀ�Ѷ��еȣ�ע����������Ի�ѧƽ���ƶ���Ӱ���Լ�ƽ��״̬���жϽǶȣ�

| A�� | �ڱ�״���£�1molˮ��1molH2�������Լ��22.4L | |
| B�� | 2gH2��44gCO2�������� | |
| C�� | 1molij��������Ϊ22.4L���������һ�����ڱ�״�� | |
| D�� | �ڱ�״���£�1gH2��11.2LO2�����ʵ������ |
| t/min | 10 | 20 | 30 | 40 | 50 | 60 |
| n��CH3OH��/mol | 0.080 | 0.120 | 0.150 | 0.168 | 0.180 | 0.180 |
��2�����¶��·�Ӧ�Ļ�ѧƽ�ⳣ����ֵΪ2.25��104 ��
��3��H2��ƽ��ת������90%��
��4����ʵ����500���£�����ƽ��ʱn��CH3OH��=0.160mol��������Ӧ�Ƿ��ȣ�����ȡ������ȡ�����Ӧ��
�ٽ�С����Ͷ�����ʯ����Һ��ˮ�У���Ӧ����Һ���
�ڽ���Ͷ��ϡ�����У�������ˮ��Ӧ���������ᷴӦ
������ˮ�����з�Ӧʱ���¶ȸ�ȼ�ա�
������������Ӧ�IJ���Ϊ�������ƣ�
| A�� | ֻ�Т٢� | B�� | ֻ�Тڢ� | C�� | ֻ�Тڢۢ� | D�� | �٢ڢۢ� |
���������� ���Ȼ�����Һ ��ͭ ������ �ݶ�����̼ �����ᱵ��
| A�� | �٢ڢ� | B�� | �٢ڢ� | C�� | �ۢ� | D�� | �٢� |
| A�� | ��Ѫ�� | B�� | ҹä֢ | C�� | �������� | D�� | ���� |
| A�� | ���³�ѹ�£�1mol���鹲�õ��Ӷ���Ϊ6NA | |
| B�� | 1mol��ȩ����������Cu��OH��2����Һ��Ӧ��ת�Ƶ�����ĿΪ2NA | |
| C�� | ��״���£�1L������ȼ�պ�������̬����ķ�����Ϊ$\frac{8}{22.4}$NA | |
| D�� | 0.1mol�����0.1mol������ȫȼ�����ĵ�O2��������Ϊ0.2 NA |
| A�� | һ��D2O����������������Ϊ8 | B�� | NH3�ĽṹʽΪ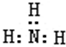 | ||
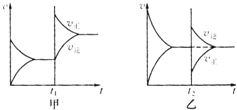
| C�� | HCl�ĵ���ʽΪ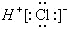 | D�� | ���ȶ��ԣ�H2S��HF |
 ��һ�������£��з�ӦxA+yB?zC��
��һ�������£��з�ӦxA+yB?zC��