��Ŀ����
����Ŀ��̼��Ԫ�أ�C��Si��Ge��Sn��Pb���ĵ��ʼ��仯�������������������й㷺��Ӧ�á��ش��������⣺
��1����̬Snԭ���У��������ռ�ݵ�����ܼ�����Ϊ__________�����ܼ����е�ԭ�ӹ����Ϊ___________________��
��2��Ge�������о�����ͽṹ��Ge�����ľ�������Ϊ_____________��Ge��ͬ���ڵ� As��Se��Ƚϣ���һ�������ɴ�С��˳����_______________��
��3�����ϱ�������������̼(C2O2)�ǽ��Ǵ������һ����ɲ��֣�������в�����״�ṹ��ÿ��ԭ�Ӿ�����8�����ȶ��ṹ��C2O2��̼ԭ�ӵ��ӻ���ʽΪ______________�������ЦҼ���м��ĸ�����Ϊ______________________��
��4��̼����ľ���ṹ�����ڽ��ʯ����ͼ��ʾ����1��̼���辧����ռ_______��̼ԭ�ӣ��������辧������С���ϵ�ԭ�Ӹ���֮��Ϊ_____________��

��5��ʯī������������Ҫԭ����__________________________��
��6�������Ľṹ�����ڽ��ʯ����֪���������������ԭ��֮��ľ���Ϊa cm����辧����ܶ�Ϊ_____________g��cm-3���ú���a�Ĵ���ʽ��ʾ����NA��ʾ����٤������ֵ����
���𰸡� 5p 3 ԭ�Ӿ��� As>Se>Ge sp 1��1 4 2��1 ʯī�������Ƭ��ṹ��Ƭ��֮�俿���ķ��»�����ϣ����Ի��� ![]()
����������1��Sn�ǵ������ڵ���AԪ�أ����̬Snԭ���У��������ռ�ݵ�����ܼ�����Ϊ5p�����ܼ����е�ԭ�ӹ����Ϊ3����2��Ge�������о�����ͽṹ�����Ge�����ľ�������Ϊԭ�Ӿ��塣ͬ�����������ҵ�һ����������������As��5p������Ӵ��ڰ����״̬���ȶ���ǿ����Ge��ͬ���ڵ� As��Se��Ƚϣ���һ�������ɴ�С��˳����As>Se>Ge����3�������в�����״�ṹ��ÿ��ԭ�Ӿ�����8�����ȶ��ṹ�����C2O2�ĽṹʽΪO=C=C=O����̼ԭ�ӵ��ӻ���ʽΪsp��˫������1��������1��������ɣ���˷����������������ĸ�����Ϊ1:1����4������̼����ľ���ṹ���ж�1��̼���辧����ռ8��1/8+6��1/2=4��̼ԭ�ӣ��������辧������С�Ļ���12��ԭ�ӣ�����ÿ����ԭ�ӱ�12�������У����ÿ����ֻռ�иù�ԭ�ӵ�1/12����Ϊÿ����С������6����ԭ�ӣ�����ÿ����С��ƽ��ӵ�еĹ�ԭ����Ϊ6��1/12=0.5��������ΪSiO2�������ɹ�ԭ�Ӻ���ԭ�Ӱ�1��2�ı�������ɣ����ÿ����С��ƽ��ӵ�е���ԭ�ӵ���ĿΪ0.5��2=1�������Զ������辧������С���ϵ�ԭ�Ӹ���֮��Ϊ2:1����5������ʯī�������Ƭ��ṹ��Ƭ��֮�俿���ķ��»�����ϣ����Ի���������ʯī����������6��������Si����=8��1/8+6��1/2+4=8���ʾ�������Ϊ8��28/NAg���辧����ܶ�Ϊ��gcm-3�����ⳤ=  �����Խ��߳���Ϊ
�����Խ��߳���Ϊ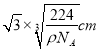 ���������������ԭ��֮��ľ���Ϊ
���������������ԭ��֮��ľ���Ϊ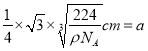 �����
�����![]() g��cm-3��
g��cm-3��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�����Ŀ��ij����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ڿ�����������CO2�����ʣ���NaCl��NaClO�����������¿ɷ�����Ӧ��ClO- + Cl- + 2H+ = Cl2��+ H2O��ijѧϰС����̽��������Һ�ı��������
(1)ȡ��������Һ�����Թ��У���������һ��Ũ�ȵ����ᣬ������ų���ͨ������װ�ü�������ijɷֿ����ж�����Һ�Ƿ���ʡ�

ѧϰС���о�����Ϊ����������������֣��ף����ֱ��ʣ��ң�δ���ʣ�����______��
Ϊ����֤����Ϊ�ף����������ʵ�鷽������ѡ�Լ���
a.98%��Ũ���� b.1%��Ʒ����Һ c.1.0 mol��L-1��KI-������Һ
d.1.0 mol��L-1 ��NaOH��Һ e.����ʯ��ˮ f.����NaCl��Һ
�����Լ� | Ԥ������ͽ��� |
�Թ�A�м�����______(����ţ��� �Թ�B�м�1%Ʒ����Һ�� �Թ�C�м�______(�����)�� | ��A��________�� B��________�� C��________����׳����� |
(2)�õζ����ⶨ����Һ��NaClO��Ũ�ȡ�
����ʹ�õζ���֮ǰ���Ƚ��еIJ�����_____________________��
����ȡ25.00 mL����Һ������ƿ�У����������a mol��L-1 Na2SO3��Һv1 mL������Ӧ�Ļ�ѧ����ʽΪ��NaClO + Na2SO3 = NaCl+ Na2SO4����b mol��L-1���������ữ��KMnO4��Һװ��_________(����������)�У��ζ�ʣ���Na2SO3��Һ����Ӧ�Ļ�ѧ����ʽΪ��_______________������Һ��__________(��ζ��յ�����)ֹͣ�ζ�����¼���ݡ�
���ظ������ζ�����2�Σ�ƽ����������KMnO4��Һv2 mL��������Һ��NaClO��Ũ��Ϊ_______mol��L-1(�ú�a��b��v1��v2�Ĵ���ʽ��ʾ)��