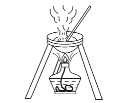
��Ŀ����
����Ŀ��ijѧϰС����ʵ������ȡ������������Ҫ��������:

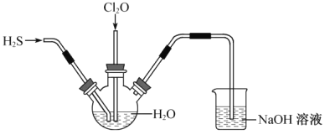
������3 mL�Ҵ���2 mLŨ�����2 mL����Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ò�������Һ����С����ȼ���3~5min��
�۴��Թ����ռ���һ���������ֹͣ���ȣ������Թ���������Ȼ���÷ֲ㡣
�ܷ��������������ϴ�ӡ����
��ش�:
��1��װ�������θ���ܣ����������������⣬��һ��Ҫ����________
��2������ڰ�װ��ʵ��װ�ã�����ҩƷǰ��Ӧ���________
��3��д����ȡ���������Ļ�ѧ����ʽ:________
��4������ʵ���б���̼������Һ��������_________������ĸ����
a �к����Ტ�����Ҵ� b �к�������Ҵ�
c ���������������ܽ� d ����������������������
��5��������Թ�����ǰ�����۲쵽��������_______�����Թ����з��������������ʵ�����������________��
���𰸡���ֹ���� װ�õ������� CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ac ��ǰ�Թ����е�Һ���ϲ���ɫ���²��ɫ����������壬����Һ��ֲ㣬�²�Һ���ɫ��dz ��Һ
CH3COOCH2CH3+H2O ac ��ǰ�Թ����е�Һ���ϲ���ɫ���²��ɫ����������壬����Һ��ֲ㣬�²�Һ���ɫ��dz ��Һ
��������
������Ҵ���Ũ����������·���������Ӧ��������Ӧ��ʵ������ȥ�ǻ���ȥ�⣬��Ϊ������Ҵ�������̼���ƣ�����ܵ����ã�һ�������������ã����Ƿ����������ã���Ϊ������������˼�ҩƷǰ��Ҫ����װ�õ������ԣ����������л���������Ҵ����Ҵ�������ˮ��������Ժ�̼���Ʒ�Ӧ�����̼���Ƶ������dz�ȥ���ᡢ�����Ҵ������������������ܽ�ȣ����������Dz�����ˮ��Һ�壬���ܶ�С��ˮ�����÷�Һ�ķ������롣
��1����Ϊ������Ҵ�������̼���ƣ�װ�������θ���ܣ����������������⣬��һ��Ҫ���÷�ֹ�������ʴ�Ϊ����ֹ������
��2����Ϊ��������������ڰ�װ��ʵ��װ�ã�����ҩƷǰ��Ӧ���װ�õ������ԣ��ʴ�Ϊ��װ�õ������ԣ�
��3��������Ҵ���Ũ����������·���������Ӧ��������Ӧ��ʵ������ȥ�ǻ���ȥ�⣬��ȡ���������Ļ�ѧ����ʽ:CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��4�����������л���������Ҵ����Ҵ�������ˮ��������Ժ�̼���Ʒ�Ӧ�����̼���Ƶ������dz�ȥ���ᡢ�����Ҵ������������������ܽ�ȣ���ѡ��ac��ȷ���ʴ�Ϊ��ac��
��5��������Թ�����ǰ�����۲쵽�������ǣ���ǰ�Թ����е�Һ���ϲ���ɫ���²��ɫ����������壬����Һ��ֲ㣬̼���������ᷴӦ�����Ա������²�Һ���ɫ��dz�����������Dz�����ˮ��Һ�壬���ܶ�С��ˮ�����Թ����з��������������ʵ����������Ƿ�Һ���ʴ�Ϊ����ǰ�Թ����е�Һ���ϲ���ɫ���²��ɫ����������壬����Һ��ֲ㣬�²�Һ���ɫ��dz����Һ��

����Ŀ��Ϊ�ⶨ![]() ��Ʒ�Ĵ��ȣ��������ܽ�6.300 g��Ʒ��������250 mL��ȡ25.00 mL��Һ����
��Ʒ�Ĵ��ȣ��������ܽ�6.300 g��Ʒ��������250 mL��ȡ25.00 mL��Һ����![]() ����Һ�ζ����յ㡣�ظ�ʵ�飬�������£�
����Һ�ζ����յ㡣�ظ�ʵ�飬�������£�
��� | �ζ�ǰ����/mL | �ζ��յ����/mL |
1 | 0.00 | 19.98 |
2 | 1.26 | 22.40 |
3 | 1.54 | 21.56 |
��֪��![]()
�������ʲ��μӷ�Ӧ��
����Ʒ��![]() ������������________%������С�����һλ����
������������________%������С�����һλ����
д����Ҫ������̣�________��