��Ŀ����
��8�֣���1��ʵ�����������Ȼ�����Һʱ���������������ǣ�ԭ�����ӷ���ʽ��ʾΪ ������������������Ϊ��ֹ��������������������ʱ������
(2)��������2���Ȼ�ѧ��Ӧ����ʽ��
FeO(s)+CO(g)=" Fe(s)+" CO2(g) ��H= �D218kJ��mol
Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H= +640.5kJ��mol
д��CO���廹ԭFe3O4����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
______________
��4�������£������ʵ�����Ũ�ȵĢٰ�ˮ ��NH4HSO4 ��NH4Cl ��(NH4)2CO3
��(NH4)2SO4��Һ�У�c(NH4+)�ɴ�С��˳��Ϊ_____________________________(�����)
(2)��������2���Ȼ�ѧ��Ӧ����ʽ��
FeO(s)+CO(g)=" Fe(s)+" CO2(g) ��H= �D218kJ��mol
Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H= +640.5kJ��mol
д��CO���廹ԭFe3O4����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
______________
��4�������£������ʵ�����Ũ�ȵĢٰ�ˮ ��NH4HSO4 ��NH4Cl ��(NH4)2CO3
��(NH4)2SO4��Һ�У�c(NH4+)�ɴ�С��˳��Ϊ_____________________________(�����)
��8�֣�ÿ��2�֣�1��Fe 3+ +3H2O 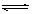 Fe(OH)3 + 3H + ����
Fe(OH)3 + 3H + ����
��2�� 4CO(g)+Fe3O4(s) = 3Fe(s)+4CO2(g)����H= ��13.5kJ/moL
��3)�ݢܢڢۢ�
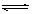 Fe(OH)3 + 3H + ����
Fe(OH)3 + 3H + ������2�� 4CO(g)+Fe3O4(s) = 3Fe(s)+4CO2(g)����H= ��13.5kJ/moL
��3)�ݢܢڢۢ�
��

��ϰ��ϵ�д�
�����Ŀ
 ��
��
 ����
����
 ��
��
