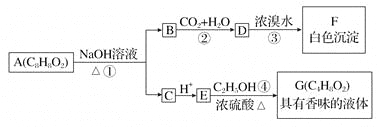
��Ŀ����
����Ŀ����1�����������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
�ٴ��������У���H2��NO3����ԭΪN2��һ��ʱ�����Һ�ļ���������ǿ����÷�Ӧ���ӷ���ʽΪ____��
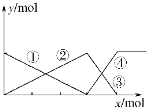
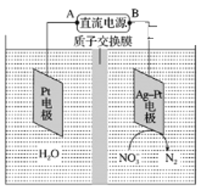
�ڵ绯ѧ����NO3����ԭ����ͼ��ʾ����Դ����Ϊ________���A���� B���������ܷ�Ӧ4NO3-+4H+=5O2+2N2+2H2O����������ӦʽΪ_______��
���ܷ�����ӽ���Ĥ��Ϊ�����ӽ���Ĥ____________����ܡ����ܡ�����
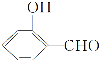
��2���о�����ȿ���CO��CO2��Ӧ�öԹ�����̬������������Ҫ�����塣
��CO��O2��Ƴ�ȼ�ϵ�أ���KOH��ҺΪ���Һ�����õ�صĸ�����ӦʽΪ______��
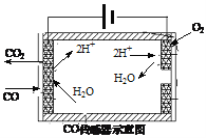
�ڲ�������β���е�COŨ�ȳ��õ绯ѧ����������������ͼ��ʾ�������������ĵ缫��ӦΪ_____________________��
���𰸡�5H2+2NO3-==N2��+2OH-+4H2O A 4NO3-+20e-+24H+=2N2��+12H2O ���� CO-2e-+4OH-=CO32-+2H2O CO-2e-+H2O=CO2+2H+
��������
��1���ٴ��������У���H2��NO3����ԭΪN2��һ��ʱ�����Һ�ļ���������ǿ������ʾ������OH-����÷�Ӧ���ӷ���ʽΪ5H2+2NO3-��N2��+2OH-+4H2O��
�ڴӵ绯ѧ����NO3����ԭ��ͼ�п��Կ�����NO3-ת��ΪN2���˵缫�õ��ӣ�Ϊ�������Ӷ��ó�BΪ��������Դ����ΪA�����ܷ�Ӧ4NO3-+4H+=5O2+2N2+2H2O����������ӦʽΪ4NO3-+20e-+24H+=2N2��+12H2O��
����ΪNO3- ת��ΪN2��Ҫ�����Ի����½��У���Ӧ�����H+��Ҫ�����ṩ�����Բ��ܰ����ӽ���Ĥ��Ϊ�����ӽ���Ĥ��
��2����CO��O2��Ƴ�ȼ�ϵ�أ���KOH��ҺΪ���Һ�����õ�صĸ�����ӦʽΪCO-2e-+4OH-=CO32-+2H2O��
�ڲ�������β���е�COŨ�ȳ��õ绯ѧ���������������ͼ�п��Կ���������CO��H2Oת��ΪCO2��H+�����������ĵ缫��ӦΪCO-2e-+H2O=CO2+2H+��

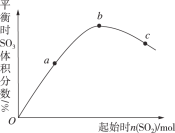
����Ŀ����һ���2L���ܱ������м��뷴Ӧ��N2��H2���������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
���ʵ���/ mol ʱ��/min | n(N2) | n(H2) | n(NH3) |
0 | 1.0 | 1.2 | 0 |
2 | 0.9 | ||
4 | 0.75 | ||
6 | 0.3 |
A. 0��2 min�ڣ�NH3�ķ�Ӧ����Ϊ0.1 mol��L��1��min��1
B. 2 minʱ�� H2�����ʵ���0.3 mol
C. 4 minʱ����Ӧ�Ѵﵽƽ��״̬����ʱ�����淴Ӧ�����ʶ�Ϊ0
D. 4��6 min�ڣ�������������ӵ������ʵ�������
����Ŀ����ˮ�к��зḻ��þ��Դ��ijͬѧ����˴�ģ�⺣ˮ���Ʊ�MgO��ʵ�鷽����

ģ�⺣ˮ�е�����Ũ��/mol��L��1 | Na�� | Mg2�� | Ca2�� | Cl�� | HCO3�� |
0.439 | 0.050 | 0.011 | 0.560 | 0.001 |
ע����Һ��ij�����ӵ�Ũ��С��1.0��10��5 mol��L��1������Ϊ�����Ӳ����ڣ�ʵ������У�������Һ������䡣Ksp[CaCO3]��4.96��10��9��Ksp[MgCO3]��6.82��10��6��Ksp[Ca(OH)2]��4.68��10��6��Ksp[Mg(OH)2]��5.61��10��12������˵����ȷ���ǣ� ��
A.������XΪCaCO3
B.��ҺM�д���Mg2����������Ca2��
C.��ҺN�д���Mg2����Ca2��
D.�����������Ϊ����4.2 g NaOH���壬������YΪCa(OH)2��Mg(OH)2�Ļ����