��Ŀ����
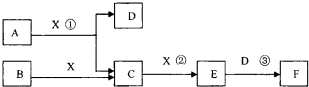
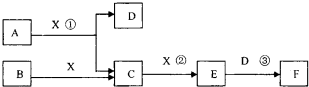
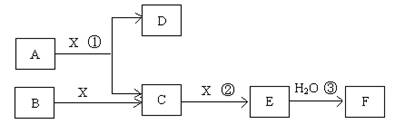
��14�֣���֪�ɶ����ڳ���Ԫ���γɵĴ�����A��B��C��D��E��F��Xת����ϵ����ͼ��ʾ��B��XΪ���ʣ�D������Ϊ��ɫҺ�壬A��B��ͬһ��Ԫ�ء���ijЩ���������ȥ��
��ش��������⣺
��1����E����ɫ���壬F��һԪǿ�ᣬ��Ӧ���ǹ�ҵ�Ʊ�F�ĵ�һ����Ӧ��
��д��A��X��Ӧ�Ļ�ѧ����ʽ�� ��
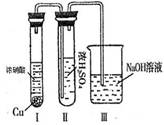
��������Ϊ��ŨH2SO4���Ը�������E����ijͬѧΪ����֤�ù۵��Ƿ���ȷ������ͼװ�ý���ʵ�顣ʵ������У�ŨH2SO4��δ�����������ݳ�����ŨH2SO4����ɫ��Ϊ����ɫ���ɴ���ó��Ľ����� ��
����֪������1 mol����E������Ӧ�۷ų�46kJ������д������E��H2O��Ӧ���Ȼ�ѧ��ʽ ��
���ڳ����£���V1L pH=a��A��Һ�м���V2L pH=b�����ᣬ��a+b=14������Ӧ����Һ��pH<7����V1��V2�Ĺ�ϵΪV1 V2����>��<����ȷ������������Һ�и������ӵ�Ũ���ɴ�С��˳������� ����д��һ��������ɣ�
��2����EΪ��ɫ��ζ���壬F�Ƕ�Ԫ���ᡣ
��E�ĵ���ʽΪ ��
�ڽ���������Eͨ������������Һ�еò�����G��G��KSP=8.1��10-9���ֽ��ó�������0.1mol/L��BaCl2��Һ�У���KSP ���������С�䣩����ʱ����ɲ�����G������������Һ�е�Ũ��Ϊ mol/L��
��1����4NH3��5O2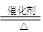 4NO��6H2O
4NO��6H2O
��NO2������Ũ�����У�Ũ����ܸ���NO2
��3 NO2��g��+ H2O��l��= 2HNO3��aq��+ NO��g���� ��H = ��138 kJ/mol
�� < ��c(Cl��) >c(Na��) >c(H��) >c(OH��)
��2����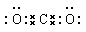 ��1�֣�
�ڲ��䣨1�֣� �� 8.1��10-8
��1�֣�
�ڲ��䣨1�֣� �� 8.1��10-8
����������1��D������Ϊ��ɫҺ�壬��D������ˮ����E����ɫ���壬F��һԪǿ�ᣬ��E��NO2��F�����ᡣ��Ӧ���ǹ�ҵ�Ʊ�F�ĵ�һ����Ӧ������A�ǰ�����X��������C��NO��B�ǵ�����
�ٸ÷�Ӧ�ǰ��Ĵ�����������ʽΪ4NH3��5O2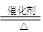 4NO��6H2O��
4NO��6H2O��
��ŨH2SO4��δ�����������ݳ�����ŨH2SO4����ɫ��Ϊ����ɫ����˵��NO2���ܽ���Ũ�����У����Ũ����ܸ���NO2���塣
�۸��������֪���÷�Ӧ���Ȼ�ѧ����ʽΪ3 NO2��g��+ H2O��l��= 2HNO3��aq��+ NO��g���� ��H = ��138 kJ/mol��
��������������Ũ����10��bmol/L����ˮ��OH����Ũ����10a��14mol/L��10��bmol/L�����ڰ�ˮ���������ˮ��Ũ��һ������10��bmol/L����Ӧ����Һ����������ˮ�����һ��С���������������ڶ���ǡ�÷�Ӧʱ����Һ�����ԣ��������ʱҺ�����ԣ���������Ũ�ȴ�С��ϵ��c(Cl��) >c(NH4��) >c(H��) >c(OH��)��c(Cl��) >c(H��)>cNH4��) >c(OH��)��
��2����EΪ��ɫ��ζ���壬F�Ƕ�Ԫ���ᣬ��E��CO2��F��̼�ᡣ
��CO2�����к���̼��˫��������ʽΪ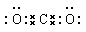 ��
��
���ܶȻ�����ֻ���¶��й�ϵ���������¶Ȳ���������£��ܶȻ��������䡣G��̼�ᱵ����������Ũ����0.1mol/Lʱ��CO32��Ũ����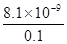 ��8.1��10-8mol/L��
��8.1��10-8mol/L��