��Ŀ����
20��ʵ����Ҫ��18mol/L Ũ��������100ml 3.0mol/L ϡ���ᣬ��ش��������⣺��1������Ũ����������16.7mL����ȡŨ�������õ���Ͳ�Ĺ����B����ѡ����ţ�
A.10ml B.20ml C.50ml D.100ml��
��2��ʵ���������Ϊ��
A�����Ƶõ���ҺС�ĵ�ת��������ƿ�У�
B����ȡ����Ũ���ᣬ���ձ���������������ˮ����ϡ�Ͳ���ȴ�����£�
C������������ƿ�м�����ˮ��Һ���̶�1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ��ײ���̶������У�
D������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ע������ƿ����������
E��������ƿ�����������ҡ�ȣ�
�����������ȷ˳��ΪBADCE������ţ���
��3����������У���ʹ������ҺŨ��ƫ�ߵ���AE������ţ���
A����ȡ����Ũ����ijͬѧ�۲�Һ��ʱ���� B��û�н��������IJ�������D
C��������ˮʱ�����������˿̶��� D������ƿʹ��ǰ�ڱ�մ��ˮ��
E������������ˮϴ����ȡŨ��������Ͳ��ϴ�ӵ�Һ��ע������ƿ��
���� ��1��������Һϡ��ǰ���������ʵ��������������Ũ������������������Ũ�������ѡ����Ͳ���
��2������Ũ�����ϡ�ͣ�����ʵ�鲽�裻
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{V}$�����жϣ�
��� �⣺��1��Ũ����100mL3.0mol/L��ϡH2SO4����ѡ��100mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL����xmL��18mol/L=100mL��3.0mol/L����ã�x��16.7mL������Ӧ��ȡ��Ũ���������16.7mL����ȡŨ�������õ���Ͳ�Ĺ����20mL��
�ʴ�Ϊ��16.7mL�� B��
��2������Ũ�����ϡ�ͣ�����ʵ�鲽�裬������ȡŨ���Ტϡ�ͣ�Ȼ��ת�Ƶ�����ƿ��ϴ���ձ��Ͳ����������ݺ�ҡ�ȣ��ʴ�Ϊ��BADCE��
��3��A����ȡ����Ũ����ijͬѧ�۲�Һ��ʱ���ӣ���ȡŨ�������ƫ��������ҺŨ��ƫ��
B��û��ϴ���ձ��Ͳ�������Ũ������٣�������ҺŨ��ƫС��
C��������ˮʱ�����������˿̶��ߣ���Һ���ƫ��������ҺŨ��ƫС��
D������ƿʹ��ǰ�ڱ�մ��ˮ�飬��������Һ��Ӱ�죻
E������������ˮϴ����ȡŨ��������Ͳ��ϴ�ӵ�Һ��ע������ƿ����ȡŨ�������ƫ��������ҺŨ��ƫ��
�ʴ𰸣�A��E��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=$\frac{n}{V}$��������ԭ����ע��Ũ�����ϡ�Ͳ٣�

| A�� | $\frac{��a+3b-2c��}{2}$kJ•mol-1 | B�� | $\frac{��a+3b+2c��}{6}$kJ•mol-1 | ||
| C�� | $\frac{��a+3b-2c��}{6}$kJ•mol-1 | D�� | $\frac{��a+3b+2c��}{3}$kJ•mol-1 |
| A�� | ������ | B�� | ¬ɪ�� | C�� | ���� | D�� | ��ķ�� |
���в��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.112 | 0.104 | 0.066 |
| ��Ҫ���ϼ� | +2 | +3 | +2 | +6��-2 | -2 |
| A�� | �⻯����ȶ���ΪH2T��H2R | B�� | ������ϡ���ᷴӦ������ΪL��Q | ||
| C�� | M3+�����Ӱ뾶��T2-�����Ӱ뾶С | D�� | L2+��R2-�ĺ����������� |
| A�� | 0.32mol NaCl��0.01mol KCl��0.12mol Na2S04 | |
| B�� | 0.02mol NaCl��0.64mol KCl��0.24mol Na2S04 | |
| C�� | 0.64mol NaCl��0.04mol KCl��0.24mol Na2S04 | |
| D�� | 0.64mol NaCl��0.02mol KCl��0.24mol Na2S04 |
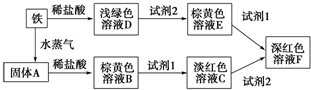 ��������ת����ϵ���ش��й����⣺
��������ת����ϵ���ش��й����⣺