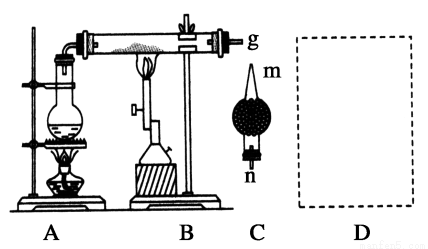
��Ŀ����
��1��ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ�� ��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ���� ��
��3������100mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
��������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
������һ���м���a mL 1mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
��1��Ca��OH��2��2NH4Cl CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3��
��2��4 mol/L ��1�֣�
��3����A13+��3NH3��H2O=Al��OH��3����3NH4+
Al��OH��3��OH��=AlO2����2H2O
0.01 mol
 �� 5
�� 5
 ��������
��������
 �����������ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ��Ca��OH��2��2NH4Cl
�����������ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ��Ca��OH��2��2NH4Cl CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3��
������c=n/V,�ð��������ʵ�����4.48L/22.4L/mol=0.2mol������������Һ���ʵ���Ũ��Ϊ��0.2mol/0.05L=4 mol/L
�Ǣ�AlCl3�백ˮ��Ӧ�����ӷ���ʽ��A13+��3NH3��H2O=Al��OH��3����3NH4+���������ټ�����������������������Һ�����ӷ���ʽΪAl��OH��3��OH��=AlO2����2H2O���������ٵ����ʵ�����Ϊ�������Ƶ����ʵ���0.01mol
������֪Al(OH)3�����ʵ���Ϊ0.01mol������NH3��H2O0.03mol������Mg2+����NH3��H2O��0.04mol-0.03mol��=0.01mol,��n��Mg2+��=0.005mol=n(SO42-����������Ҫ1mol/LBaCl2��Һ5mlʹSO42-������ȫ��
���㣺���ӷ�Ӧ����ؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�