ћвƒњƒЏ»Ё
Їм»»µƒћъƒ№”лЋЃ’ф∆шЈі”¶£ђ”–«в∆ш…ъ≥…£ђѕ÷”√»зЌЉЋщ Њ„∞÷√љш––ћъ‘ЏЄяќ¬ѕ¬”лЋЃ’ф∆шЈі”¶µƒ µ—й£ђ≤Ґ”√Љтµ•µƒЈљЈ® ’Љѓ°ҐЉм—й…ъ≥…µƒ«в∆ш°£«лїЎірѕ¬Ѕ–ќ ћв£Ї
(1)–і≥цћъ‘ЏЄяќ¬ѕ¬”лЋЃ’ф∆шЈі”¶µƒїѓ—ІЈљ≥ћ љ£Ї °£
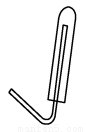
(2)Є…‘пє№CƒЏ ҐЈ≈µƒ“©∆Ј « ЇЌ (ћоїѓ—І љ)°£Є…‘пє№µƒ (ћо°∞m°±їт°∞n°±)ґЋ”лgµЉє№ѕаЅђљ”°£
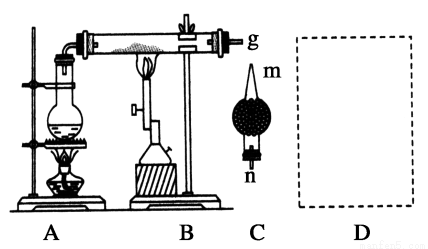
(3)‘ЏDі¶ї≠≥ц”√ ‘є№ ’Љѓ«в∆шµƒ„∞÷√ЌЉ(Ћщ–и∆дЋы“«∆ч„‘––—°‘с)°£
(4)‘х—щ”√Љтµ•µƒЈљЈ®Љм—й ’Љѓµљµƒ∆шће ««в∆ш£ђЉт ц µ—й≤ў„ч≤љ÷иЇЌѕ÷ѕу °£
(1)3Fe£Ђ4H2O(g)  Fe3O4£Ђ4H2 (2)CaCl2 CaO n
Fe3O4£Ђ4H2 (2)CaCl2 CaO n
(3)
(4)”√ƒі÷Єґ¬„° ‘є№њЏ£ђ ‘є№њЏѕт…ѕ£ђњњљьЊ∆ЊЂµ∆їр—ж£ђЋ…њ™ƒі÷Є£ђƒ№»Љ…’їтЈҐ≥ц±ђ√щ…щ£ђЋµ√ч ’Љѓµƒ «H2°£
°Њљвќц°њ
‘ћвЈ÷ќц£ЇҐ≈ћъ‘ЏЄяќ¬ѕ¬”лЋЃ’ф∆шЈі”¶µƒїѓ—ІЈљ≥ћ љ£Ї3Fe£Ђ4H2O(g)  Fe3O4£Ђ4H2 £їҐ∆„∞÷√ є”√Є…‘пє№ «Є…‘п«в∆ш÷–µƒЋЃ’ф∆ш£ђЄ…‘пє№÷–њ…„∞≤ї”лH2Јі”¶µƒЄ…‘пЉЅ£ђ»зCaCl2°ҐCaOµ»£ђЄ…‘пє№÷–∆шће”¶і”іує№љш£ђ–°є№≥ц£ђЋщ“‘nґЋ”лgµЉє№ѕаЅђљ”
Fe3O4£Ђ4H2 £їҐ∆„∞÷√ є”√Є…‘пє№ «Є…‘п«в∆ш÷–µƒЋЃ’ф∆ш£ђЄ…‘пє№÷–њ…„∞≤ї”лH2Јі”¶µƒЄ…‘пЉЅ£ђ»зCaCl2°ҐCaOµ»£ђЄ…‘пє№÷–∆шће”¶і”іує№љш£ђ–°є№≥ц£ђЋщ“‘nґЋ”лgµЉє№ѕаЅђљ”
Ґ«H2√№ґ»–°”Џњ’∆ш£ђ”¶”√ѕтѕ¬≈≈њ’Ј® ’Љѓ£ђ”√ ‘є№ ’Љѓ«в∆шµƒ„∞÷√ЌЉќ™ Ґ»Љм—й ’Љѓµљµƒ∆шће ««в∆ш£ђ≤ў„чќ™£Ї”√ƒі÷Єґ¬„° ‘є№њЏ£ђ ‘є№њЏѕт…ѕ£ђњњљьЊ∆ЊЂµ∆їр—ж£ђЋ…њ™ƒі÷Є£ђƒ№»Љ…’їтЈҐ≥ц±ђ√щ…щ£ђЋµ√ч ’Љѓµƒ «H2£ђір∞Є£Ї”√ƒі÷Єґ¬„° ‘є№њЏ£ђ ‘є№њЏѕт…ѕ£ђњњљьЊ∆ЊЂµ∆їр—ж£ђЋ…њ™ƒі÷Є£ђƒ№»Љ…’їтЈҐ≥ц±ђ√щ…щ£ђЋµ√ч ’Љѓµƒ «H2°£
Ґ»Љм—й ’Љѓµљµƒ∆шће ««в∆ш£ђ≤ў„чќ™£Ї”√ƒі÷Єґ¬„° ‘є№њЏ£ђ ‘є№њЏѕт…ѕ£ђњњљьЊ∆ЊЂµ∆їр—ж£ђЋ…њ™ƒі÷Є£ђƒ№»Љ…’їтЈҐ≥ц±ђ√щ…щ£ђЋµ√ч ’Љѓµƒ «H2£ђір∞Є£Ї”√ƒі÷Єґ¬„° ‘є№њЏ£ђ ‘є№њЏѕт…ѕ£ђњњљьЊ∆ЊЂµ∆їр—ж£ђЋ…њ™ƒі÷Є£ђƒ№»Љ…’їтЈҐ≥ц±ђ√щ…щ£ђЋµ√ч ’Љѓµƒ «H2°£
њЉµг£ЇћъЉ∞∆дїѓЇѕќпµƒ–‘÷ µ—й

 –°—Іљћ≤ƒ»Ђ≤вѕµЅ–ір∞Є
–°—Іљћ≤ƒ»Ђ≤вѕµЅ–ір∞Є –°—І э—ІњЏЋгћвњ®Ќ—њЏґш≥цѕµЅ–ір∞Є
–°—І э—ІњЏЋгћвњ®Ќ—њЏґш≥цѕµЅ–ір∞Є