��Ŀ����
����̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӡ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⡣
��1��д��CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽ ��
��֪�� �� CO(g)+H2O(g) H2(g)+CO2(g) ��H����41kJ��mol��1
H2(g)+CO2(g) ��H����41kJ��mol��1
�� C(s)+2H2(g) CH4(g) ��H����73kJ��mol��1
CH4(g) ��H����73kJ��mol��1
�� 2CO(g) C(s)+CO2(g) ��H����171kJ��mol��1
C(s)+CO2(g) ��H����171kJ��mol��1
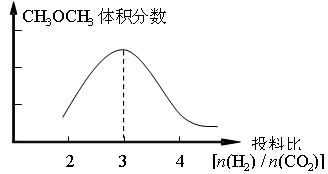
��2����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g) + 6H2(g) CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ��
CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ��
����������������ʱ��������ͼ�л���ƽ��ʱCH3OCH3�����������Ͷ�ϱ�[n(H2) / n(CO2)]�仯������ͼ��

��ij�¶��£���2.0molCO2(g)��6.0molH2(g)�����ݻ�Ϊ2L���ܱ������У���Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ��CH3OCH3(g)�����ʵ��������仯�����ͼ��ʾ�������¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ���� ��

A. P3��P2��T3��T2 B. P1��P3��T1��T3 C. P2��P4��T4��T2 D. P1��P4��T2��T3
���ں����ܱ������ﰴ�����Ϊ1:3���������̼���� ����һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ����� ��
A. ����Ӧ������������С
B. �淴Ӧ������������С
C. ��ѧƽ�ⳣ��Kֵ����
D. ��Ӧ�������ٷֺ�������
E. ���������ܶȼ�С
F. ������ת���ʼ�С
��3�������ѧ���ٴ��������ɫ��ѧ�����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״����״�������ȼ�ϵ�أ�д����ϡ����Ϊ����ʼ״�ȼ�ϵ�ظ�����Ӧʽ__ ���Դ�ȼ�ϵ����Ϊ��ӵ�Դ��ͼ��ʾ�������ͭ��Һ�������ʼʱʢ��1000mL pH��5������ͭ��Һ��25�棬CuSO4��������һ��ʱ�����Һ��pH��Ϊ1����ʱ�ɹ۲쵽�������� ����Ҫʹ��Һ�ָ�����ʼŨ�ȣ��¶Ȳ��䣬������Һ����ı仯����������Һ�м��� �����������ƣ���������ԼΪ g��

��1��д��CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽ ��
��֪�� �� CO(g)+H2O(g)
 H2(g)+CO2(g) ��H����41kJ��mol��1
H2(g)+CO2(g) ��H����41kJ��mol��1�� C(s)+2H2(g)
 CH4(g) ��H����73kJ��mol��1
CH4(g) ��H����73kJ��mol��1�� 2CO(g)
 C(s)+CO2(g) ��H����171kJ��mol��1
C(s)+CO2(g) ��H����171kJ��mol��1��2����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g) + 6H2(g)
 CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ��
CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ������������������ʱ��������ͼ�л���ƽ��ʱCH3OCH3�����������Ͷ�ϱ�[n(H2) / n(CO2)]�仯������ͼ��

��ij�¶��£���2.0molCO2(g)��6.0molH2(g)�����ݻ�Ϊ2L���ܱ������У���Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ��CH3OCH3(g)�����ʵ��������仯�����ͼ��ʾ�������¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ���� ��

A. P3��P2��T3��T2 B. P1��P3��T1��T3 C. P2��P4��T4��T2 D. P1��P4��T2��T3
���ں����ܱ������ﰴ�����Ϊ1:3���������̼���� ����һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ����� ��
A. ����Ӧ������������С
B. �淴Ӧ������������С
C. ��ѧƽ�ⳣ��Kֵ����
D. ��Ӧ�������ٷֺ�������
E. ���������ܶȼ�С
F. ������ת���ʼ�С
��3�������ѧ���ٴ��������ɫ��ѧ�����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״����״�������ȼ�ϵ�أ�д����ϡ����Ϊ����ʼ״�ȼ�ϵ�ظ�����Ӧʽ__ ���Դ�ȼ�ϵ����Ϊ��ӵ�Դ��ͼ��ʾ�������ͭ��Һ�������ʼʱʢ��1000mL pH��5������ͭ��Һ��25�棬CuSO4��������һ��ʱ�����Һ��pH��Ϊ1����ʱ�ɹ۲쵽�������� ����Ҫʹ��Һ�ָ�����ʼŨ�ȣ��¶Ȳ��䣬������Һ����ı仯����������Һ�м��� �����������ƣ���������ԼΪ g��

��1��CO2(g)+4H2(g) CH4(g)+2H2O(g) ��H����162kJ��mol��1 ��2�֣�
CH4(g)+2H2O(g) ��H����162kJ��mol��1 ��2�֣�
(2) �ٻ�ͼ����ͼ����2�֣�
��BD ��2�֣� �� B ��2�֣�
��3��CH3OH+H2O-6e-=CO2��+6H+ ��2�֣�ʯī�缫���������ݲ��������缫�ϸ���һ���ɫ���ʣ���Һ��ɫ��dz��3�֣���3��������֣�������ͭ����̼��ͭ����1�֣���4g����6.2g����1�֣�
 CH4(g)+2H2O(g) ��H����162kJ��mol��1 ��2�֣�
CH4(g)+2H2O(g) ��H����162kJ��mol��1 ��2�֣�(2) �ٻ�ͼ����ͼ����2�֣�

��BD ��2�֣� �� B ��2�֣�
��3��CH3OH+H2O-6e-=CO2��+6H+ ��2�֣�ʯī�缫���������ݲ��������缫�ϸ���һ���ɫ���ʣ���Һ��ɫ��dz��3�֣���3��������֣�������ͭ����̼��ͭ����1�֣���4g����6.2g����1�֣�
�����������1����֪���� CO(g)+H2O(g)
 H2(g)+CO2(g) ��H����41kJ��mol��1���� C(s)+2H2(g)
H2(g)+CO2(g) ��H����41kJ��mol��1���� C(s)+2H2(g) CH4(g) ��H����73kJ��mol��1���� 2CO(g)
CH4(g) ��H����73kJ��mol��1���� 2CO(g) C(s)+CO2(g) ��H����171kJ��mol��1������ݸ�˹���ɿ�֪���ۣ��١�2+�ڼ��õ�CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽO2(g)+4H2(g)
C(s)+CO2(g) ��H����171kJ��mol��1������ݸ�˹���ɿ�֪���ۣ��١�2+�ڼ��õ�CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽO2(g)+4H2(g) CH4(g)+2H2O(g) ��H����162kJ��mol��1��
CH4(g)+2H2O(g) ��H����162kJ��mol��1����2���ٸ���ͼ���֪CO2��ƽ��ת�������¶�һ������������Ͷ�ϱȵ�����������ݷ���ʽ��֪Ͷ�ϱȣ�3ʱ������ĺ�����ߣ�������ȻCO2��ƽ��ת�������¶�һ������������Ͷ�ϱȵ���������������ѵ��������ֻ����Ͷ�ϱȣ�3ʱ�������ͼ����Ա�ʾΪ���𰸡�
�ڶ��ڷ�Ӧ��2CO2(g) + 6H2(g)
 CH3OCH3(g) + 3H2O(g)������ѹǿ��ƽ��������Ӧ�����ƶ���������ѵ����ʵ�������Խ�������¶ȶ�����̼��ת���ʽ��ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ���������¶�ƽ�����淴Ӧ�����ƶ��������ѵ����ʵ�������ԽС������P1��P2��P3��P4��T1��T2��T3��T4����ѡBD��
CH3OCH3(g) + 3H2O(g)������ѹǿ��ƽ��������Ӧ�����ƶ���������ѵ����ʵ�������Խ�������¶ȶ�����̼��ת���ʽ��ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ���������¶�ƽ�����淴Ӧ�����ƶ��������ѵ����ʵ�������ԽС������P1��P2��P3��P4��T1��T2��T3��T4����ѡBD����A.����Ӧ������������С��˵����Ӧ������Ӧ�����ƶ���A����ȷ��B. �淴Ӧ������������С��˵����Ӧ���淴Ӧ�����ƶ���B��ȷ��C.��ѧƽ�ⳣ��Kֵ����˵��ƽ��������Ӧ�����ƶ���C����ȷ��D. ��Ӧ�������ٷֺ�������˵����Ӧ������Ӧ�����ƶ���D����ȷ��E. �ܶ��ǻ�����������������ݻ��ı�ֵ���ڷ�Ӧ�������������ݻ�ʼ���Dz���ģ���˻��������ܶ�ʼ�ղ��䣬E����ȷ�� F. ������ת���ʼ�С����ƽ�ⲻһ�����淴Ӧ�����ƶ�������ͨ��������������ת����Ҳ�ϵͣ�F����ȷ����ѡB��
��3��ԭ����и���ʧȥ���ӷ���������Ӧ������ϡ����Ϊ����ʼ״�ȼ�ϵ���м״��ڸ���ͨ�룬������ӦʽΪCH3OH+H2O-6e-=CO2��+6H+������װ��ͼ��֪��ʯī����������Һ�е�OH���ŵ�ų�������������������Һ�е�ͭ���ӷŵ�����ͭ������ʵ��������ʯī�缫���������ݲ��������缫�ϸ���һ���ɫ���ʣ���Һ��ɫ��dz����������������ͭ��ϡ���ᣬ������Ҫʹ��Һ�ָ�����ʼŨ�ȣ��¶Ȳ��䣬������Һ����ı仯����������Һ�м�������ͭ��̼��ͭ����Һ�������ӵ����ʵ�����0.1mol/L��1L��0.1mol������ݷ���ʽ2CuSO4��2H2O
 2H2SO4��2Cu��O2����֪����Ҫ����ͭ�����ʵ�����0.1mol��2��0.05mol��������0.05mol��80g/mol��4.0g����̼��ͭ����������0.05mol��124/mol��6.2g��
2H2SO4��2Cu��O2����֪����Ҫ����ͭ�����ʵ�����0.1mol��2��0.05mol��������0.05mol��80g/mol��4.0g����̼��ͭ����������0.05mol��124/mol��6.2g��
��ϰ��ϵ�д�
�����Ŀ
 CO2(g)+H2(g)��t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c(H2)=0.12mol��L-1������¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
CO2(g)+H2(g)��t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c(H2)=0.12mol��L-1������¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
 CH3OH��g����
CH3OH��g���� O2��g��=H2O��g������H����241.8 kJ/mol��
O2��g��=H2O��g������H����241.8 kJ/mol�� ����ʾ�辧���е�һ��ԭ��,����������Ķ����á�
����ʾ�辧���е�һ��ԭ��,����������Ķ����á�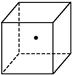
 Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol��
Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol��  N2��g��+ CO2��g������H= Q kJ?mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2��g��+ CO2��g������H= Q kJ?mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�