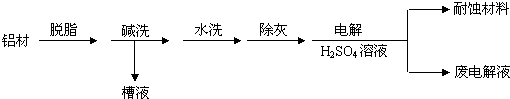
��Ŀ����
15���������Ʊ��治������ʣ�����һƿ�ѱ��ʵĹ������ƣ�ij����С���ͬѧ������ʺ�����ʿ�����̼���ƣ����ǽ�������ʵ�飺��1��ȡ������Ʒ���Թ��У�����ˮ���Ƴ���Һ���μ��Ȼ������Ȼ�����Һ���۲쵽�а�ɫ����������˵����Ʒ�к���Na2CO3��
��2��Ϊ�˴��Բⶨ�������Ƶ�������������С��ͬѧ��ȡag��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������
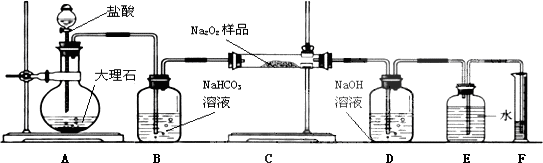
��ͼ�е�E��F��������װ�ã������ⶨO2�������
�ٽ��������Ӻ��Ժ�����еĵ�һ�������Ǽ��װ�õ������ԣ�
��д��װ��B��C�з�����Ӧ�Ļ�ѧ����ʽ��
װ��B��NaHCO3+HCl=CO2��+H2O+NaCl��
װ��C��2CO2+2Na2O2=2Na2CO3+O2��2H2O+2Na2O2=4NaOH+O2����
��NaOH�����������ջ�������ж�����̼�����ڲ��������������
�ܴ���Ӧֹͣʱ�����������������ȷ����������BCA������ţ���
A����ȷ������Ͳ��ˮ�������
B��ʹ��Ͳ���ƿ�����嶼��ȴ�����£�
C��������Ͳ�߶ȣ�ʹ���ƿ����Ͳ��Һ��߶���ƽ
�ݽ���������������ɱ�״�������Ϊb mL������Ʒ�й������Ƶ���������Ϊ$\frac{39V}{56a}$%��
���� ��1��̼������Ӻ�Ca2+��Ba2+����̼��ƻ�̼�ᱵ������
��2������ʵ��ԭ����֪����ʵ��װ����������μӷ�Ӧ������ʵ�鿪ʼʱҪ�ȼ��װ�õ������ԣ�Aװ������ʹ���ʯ��Ӧ��ȡ������̼��Bװ��������ȥ�ӷ����Ȼ��⣬Cװ���й������ƺͶ�����̼��Ӧ��Dװ����������δ��Ӧ�Ķ�����̼��E��F������ȡ��������������������Ͳ��ȡҺ�����ʱ��Ҫ����ȴ�����¼��ͼ���ƿҺ����ƽȷ������˳��������������������������ʵ�������ϻ�ѧ����ʽ����õ������������ʵ������õ��������Ƶ�����������
��� �⣺��1��̼������Ӻ�Ca2+��Ba2+����̼��ƻ�̼�ᱵ�������μ��Ȼ������Ȼ������ɰ�ɫ������֤����̼������ӣ�
�ʴ�Ϊ���Ȼ������Ȼ��ƣ�
��2���ٸ���ʵ��ԭ����֪����ʵ��װ����������μӷ�Ӧ������ʵ�鿪ʼʱҪ�ȼ��װ�õ������ԣ�
�ʴ�Ϊ�����װ�õ������ԣ�
��B�ж�����̼���Ȼ����̼�����Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ����Ӧ�ķ���ʽΪ��NaHCO3+HCl=CO2��+H2O+NaCl��Cװ���й������ƺͶ�����̼�Լ�ˮ��Ӧ����̼���ơ��������ƺ���������Ӧ����ʽΪ2CO2+2Na2O2=2Na2CO3+O2��2H2O+2Na2O2=4NaOH+O2����
�ʴ�Ϊ��NaHCO3+HCl=CO2��+H2O+NaCl��2CO2+2Na2O2=2Na2CO3+O2��2H2O+2Na2O2=4NaOH+O2����
���������ƺͶ�����̼��Ӧ�����ն�����̼�����ڲ���������������ʴ�Ϊ�����ջ�������ж�����̼�����ڲ��������������
��ֱ�Ӷ�ȡ�������������ȴ�����£���ʹ��Һ��������������������Ӧ����ȴ�����£�������Ͳ����Һ��߶�ʹ֮��ͬ��ʹװ����ѹǿ�����ѹǿ��ͬ�������밼Һ�����͵���ƽ��ȡ��Ͳ��ˮ���������ȷ�Ķ�ȡ�������ʲ�������Ϊ��BCA��
�ʴ�Ϊ��BCA��
�ݲⶨ����Ͳ��ˮ�����������ɱ�״�������������ΪVmL�����ʵ���=$\frac{V��1{0}^{-3}L}{22.4L/mol}$������Ʒ�й������Ƶ���������Ϊ=$\frac{\frac{V��1{0}^{-3}}{22.4}mol��2��78g/mol}{ag}$��100%=$\frac{39V}{56a}$%��
�ʴ�Ϊ��$\frac{39V}{56a}$%��
���� ���⿼����������ɵ�ʵ��̽��������ʵ����Ʒ�����ʵ�����Ӧ�ã�װ��ͼ�ķ����ж��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

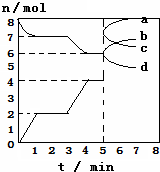 �����Ļ�ԭ��CO���ɽ�̿��CO2��Ӧ���ã��ֽ���̿��CO2�������Ϊ2L���ܱ���
�����Ļ�ԭ��CO���ɽ�̿��CO2��Ӧ���ã��ֽ���̿��CO2�������Ϊ2L���ܱ������У������½������з�Ӧ��C��s��+CO2��g��?2CO��g����H=QkJ/mol����ͼΪCO2��CO�����ʵ���n��ʱ��t�ı仯��ϵͼ������˵����ȷ�ģ�������
| A�� | 3minʱ�¶���T1���ߵ�T2����Q��0������ƽ��ʱK��T2����K��T1��=14�s3 | |
| B�� | �������ڵ�ѹǿ����ʱ���÷�Ӧһ���ﵽƽ��״̬����$\frac{P��ƽ�⣩}{P����ʼ��}$��1 | |
| C�� | 0��1min��v��CO��=1mol/��L•min����1��5minʱ��v����CO��=v����CO2�� | |
| D�� | 5minʱ�ٳ���һ������CO��a��d���߷ֱ��ʾn��CO����n��CO2���ı仯 |
| A�� | ��ѧ����������ʶ���� | |
| B�� | ������ˮӦ��������ɫ�Լ�ƿ�У����������� | |
| C�� | �ڻ�ѧ��Ӧ�У��μӷ�Ӧ�ĸ����ʵ������ȵ��������ʵ���֮�� | |
| D�� | Ӣ����ѧ�Ҳ�������ԭ��ѧ˵��Ϊ������ѧ�ķ�չ�춨�˼�ʵ�Ļ��� |
| A�� | ����Cl-���ӵ��Լ��������������� | |
| B�� | ����һ�����ʵ���Ũ����Һʱ��δϴ���ձ��Ͳ������������ƫ�� | |
| C�� | ����һ�����ʵ���Ũ����Һʱ������ƿ������������ˮ����Ũ����Ӱ�� | |
| D�� | ����Һ�м���BaCl2��Һ�����ɰ�ɫ����������֤����SO${\;}_{4}^{2-}$ |
| A�� | CuO��Cu��OH��2 | B�� | SO2��Na2SO3 | C�� | Fe��FeCl2 | D�� | CaCO3��Ca��NO3��2 |

 �о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮
�о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮